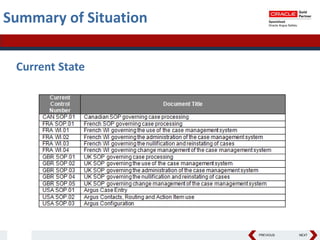
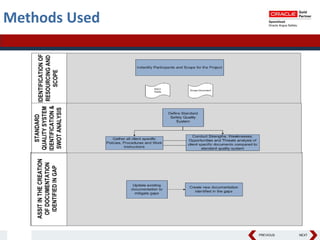
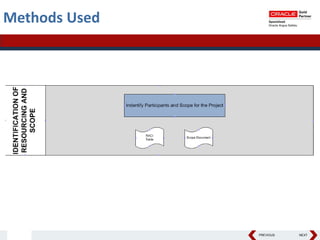
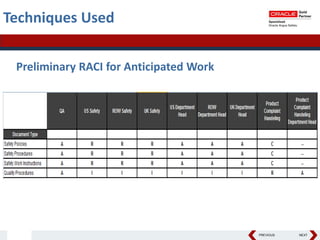
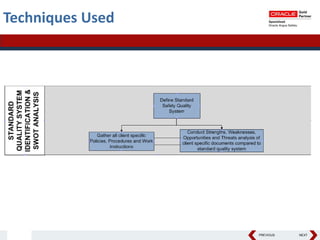
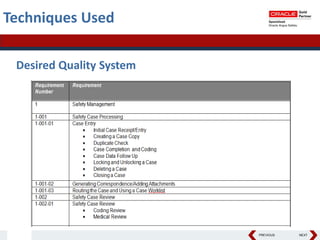
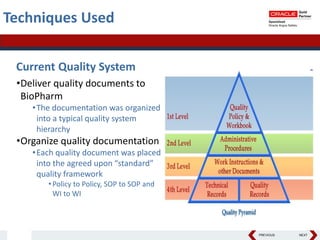
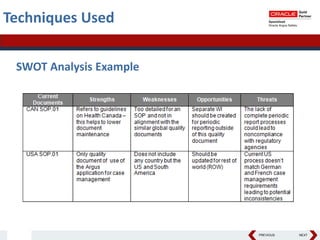
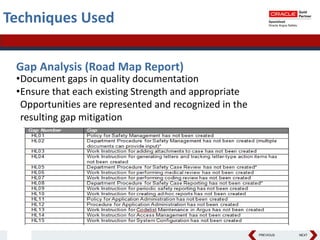
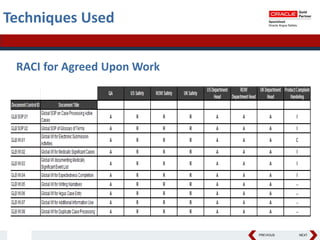
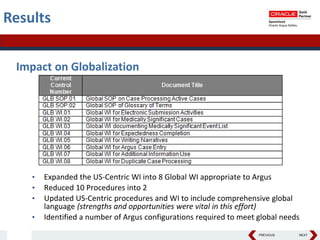
This document summarizes a project to streamline a global life sciences company's pharmacovigilance operations. The company needed to integrate three separate safety applications into its central Argus database to accommodate its acquisition of a global generics company. Methods used included a RACI diagram to define roles and responsibilities, a SWOT analysis to identify gaps between the current and desired quality systems, and iterative meetings to reach consensus on harmonized global documentation. The results expanded country-specific documents into a comprehensive global framework and reduced procedures, facilitating the company's globalization goals. Lessons learned included ensuring accurate quality documents for analysis and using a gap report to focus process re-engineering efforts.