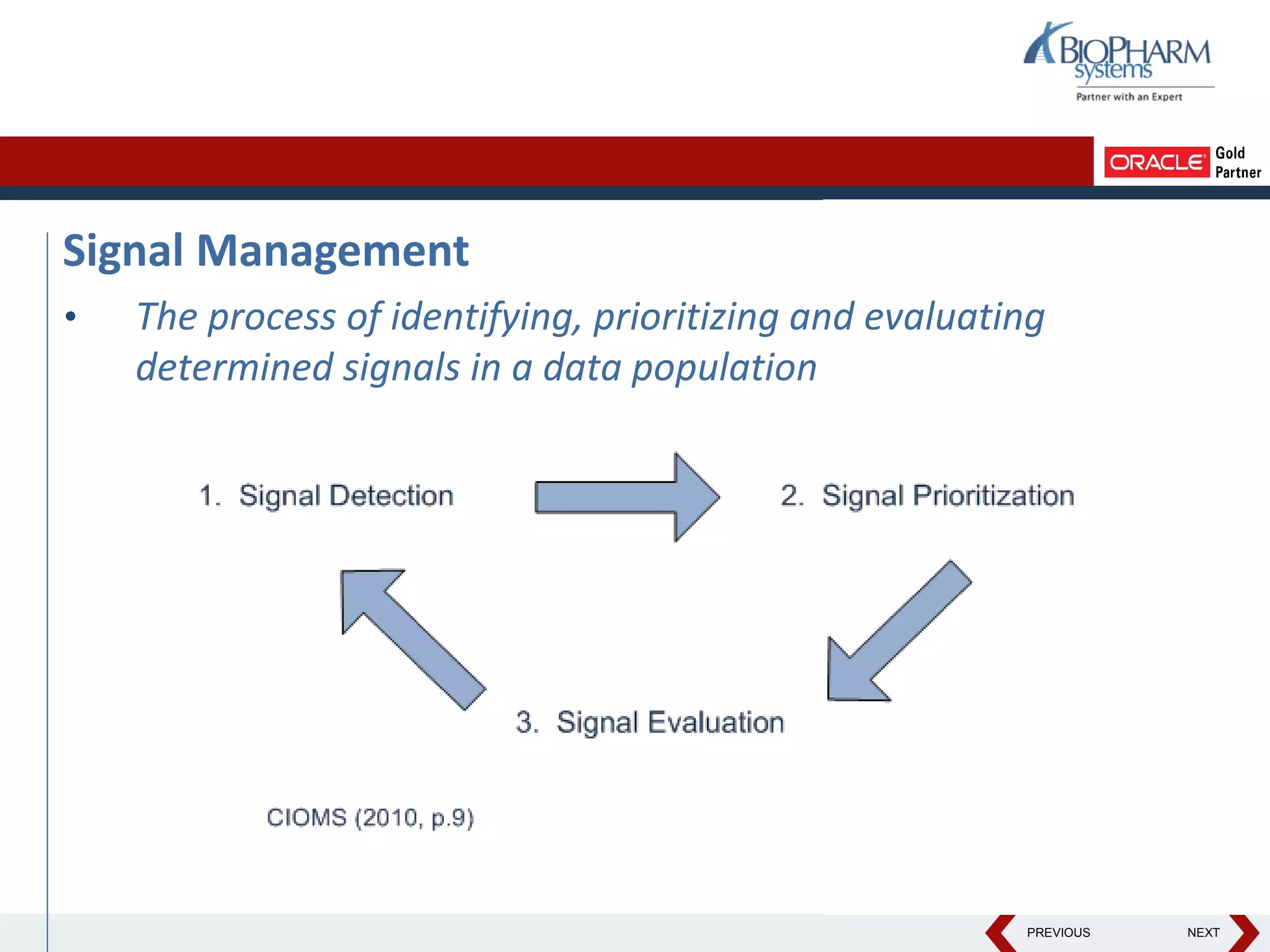
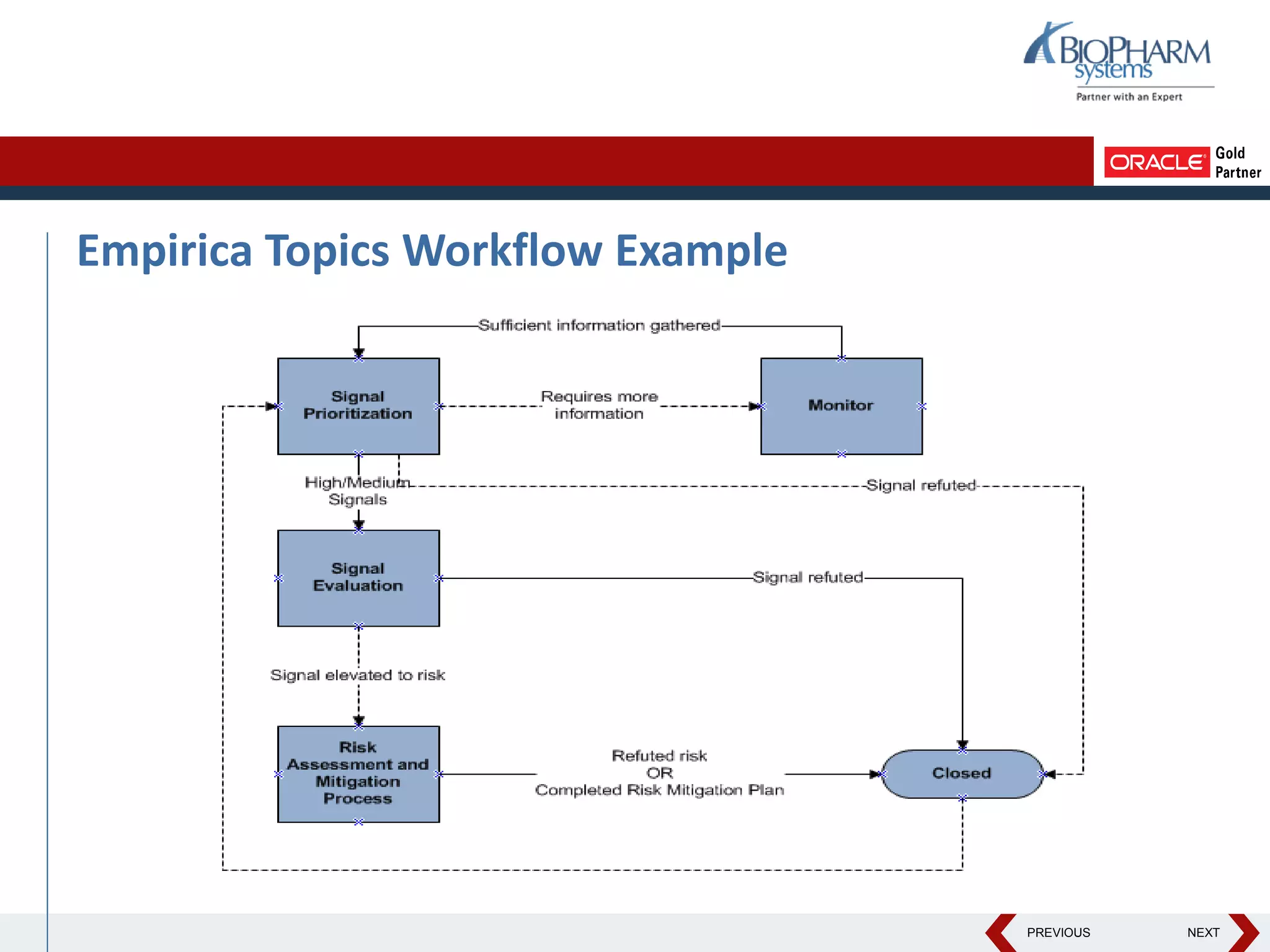
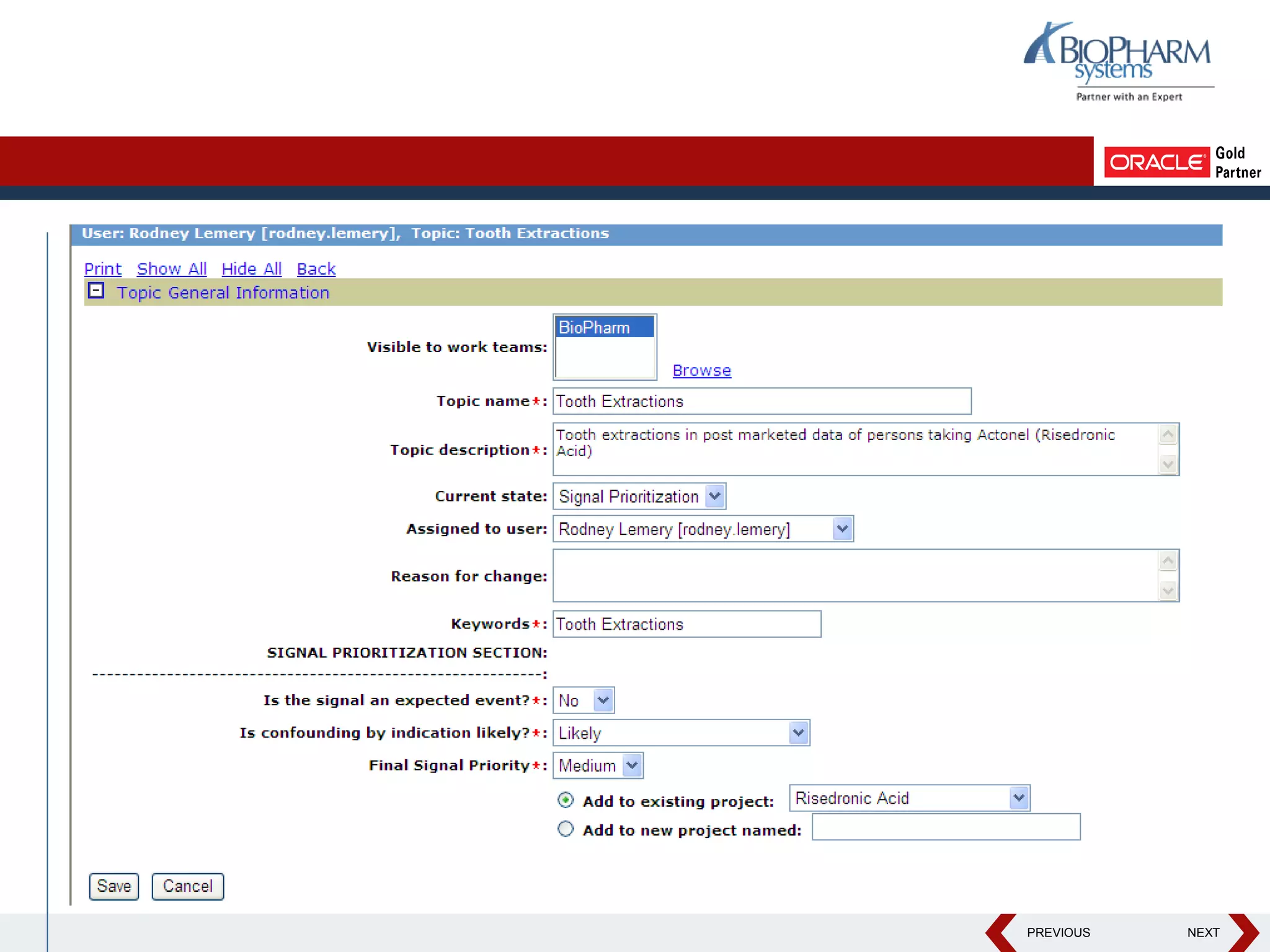
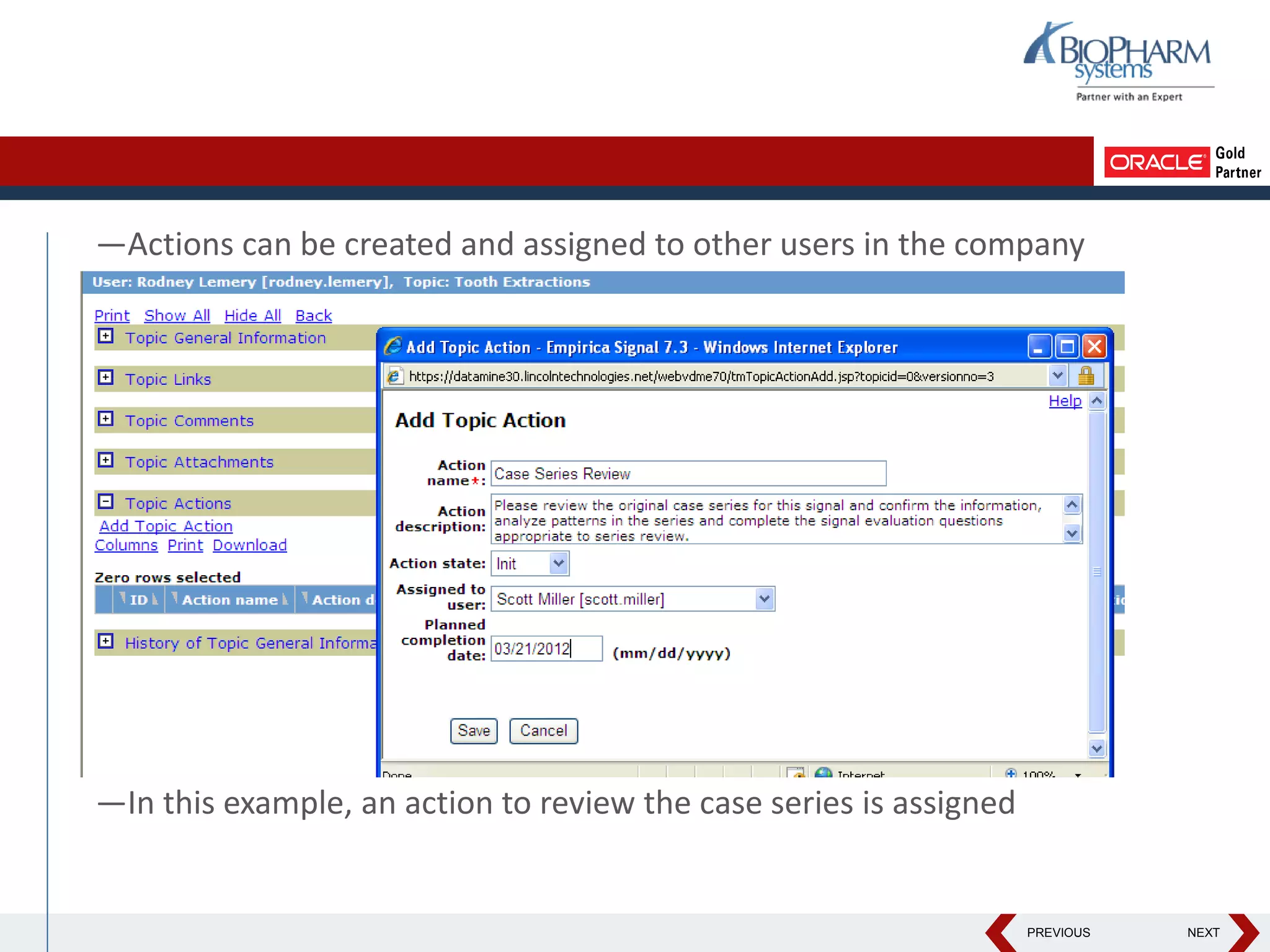
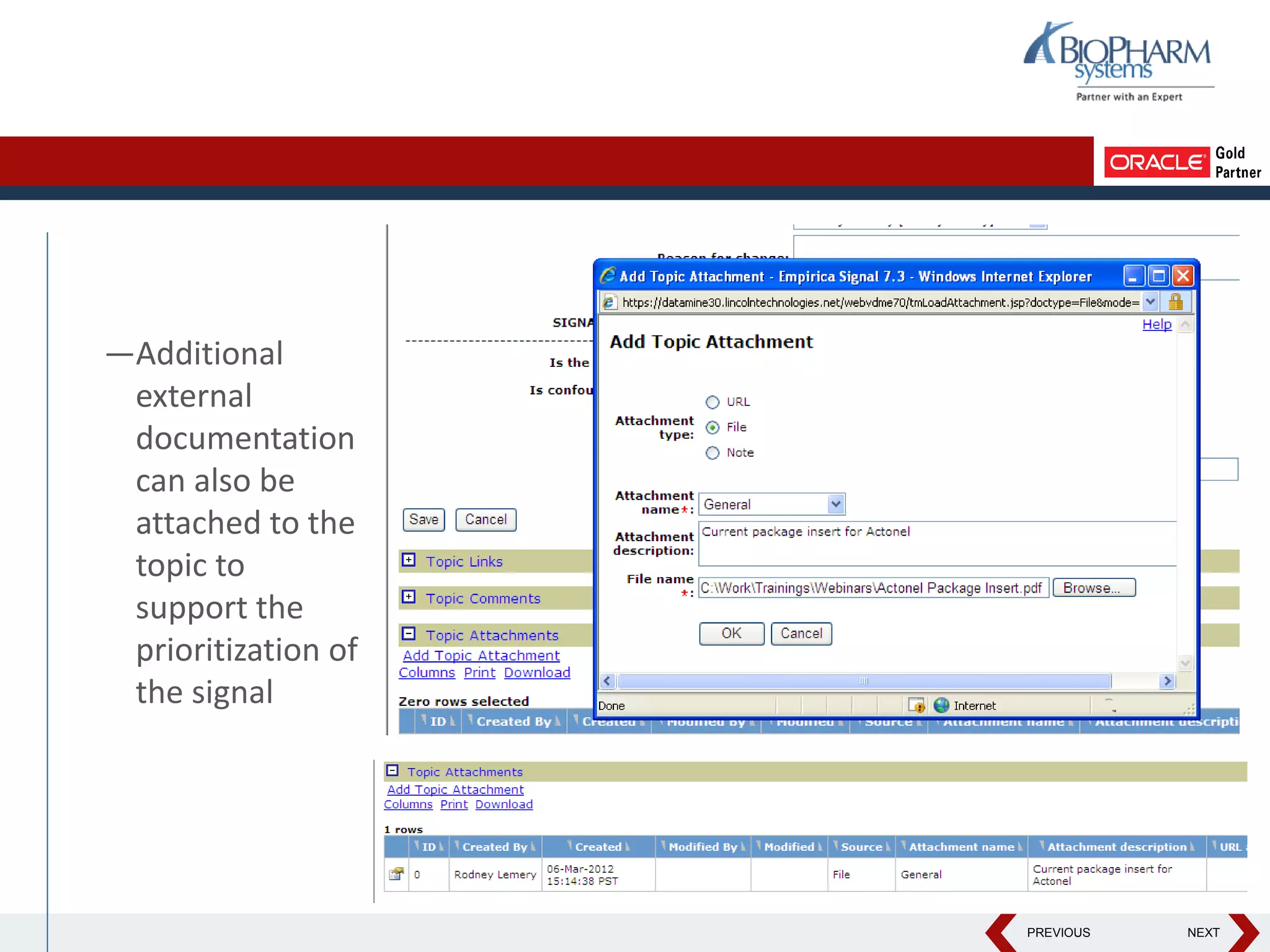
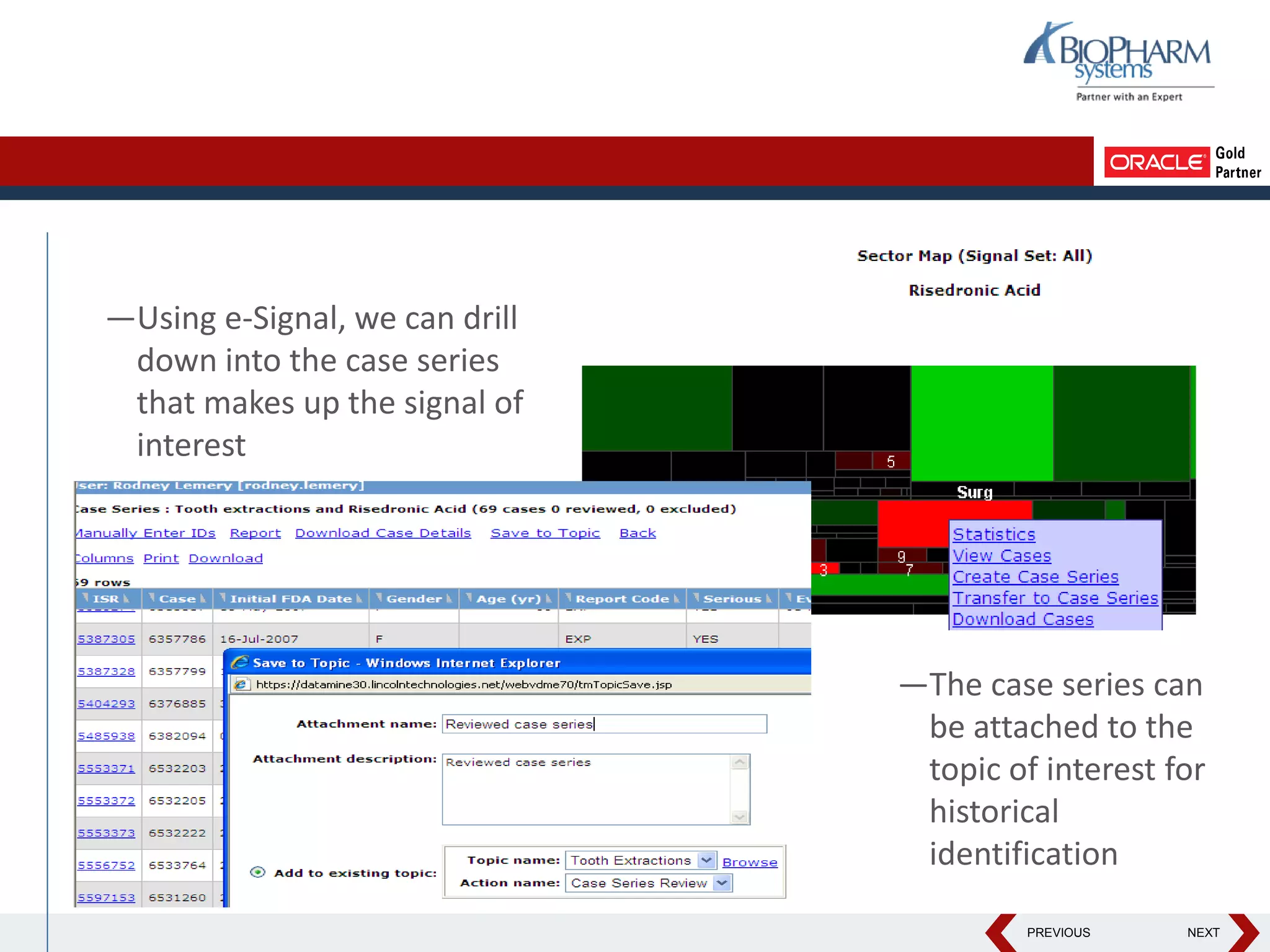
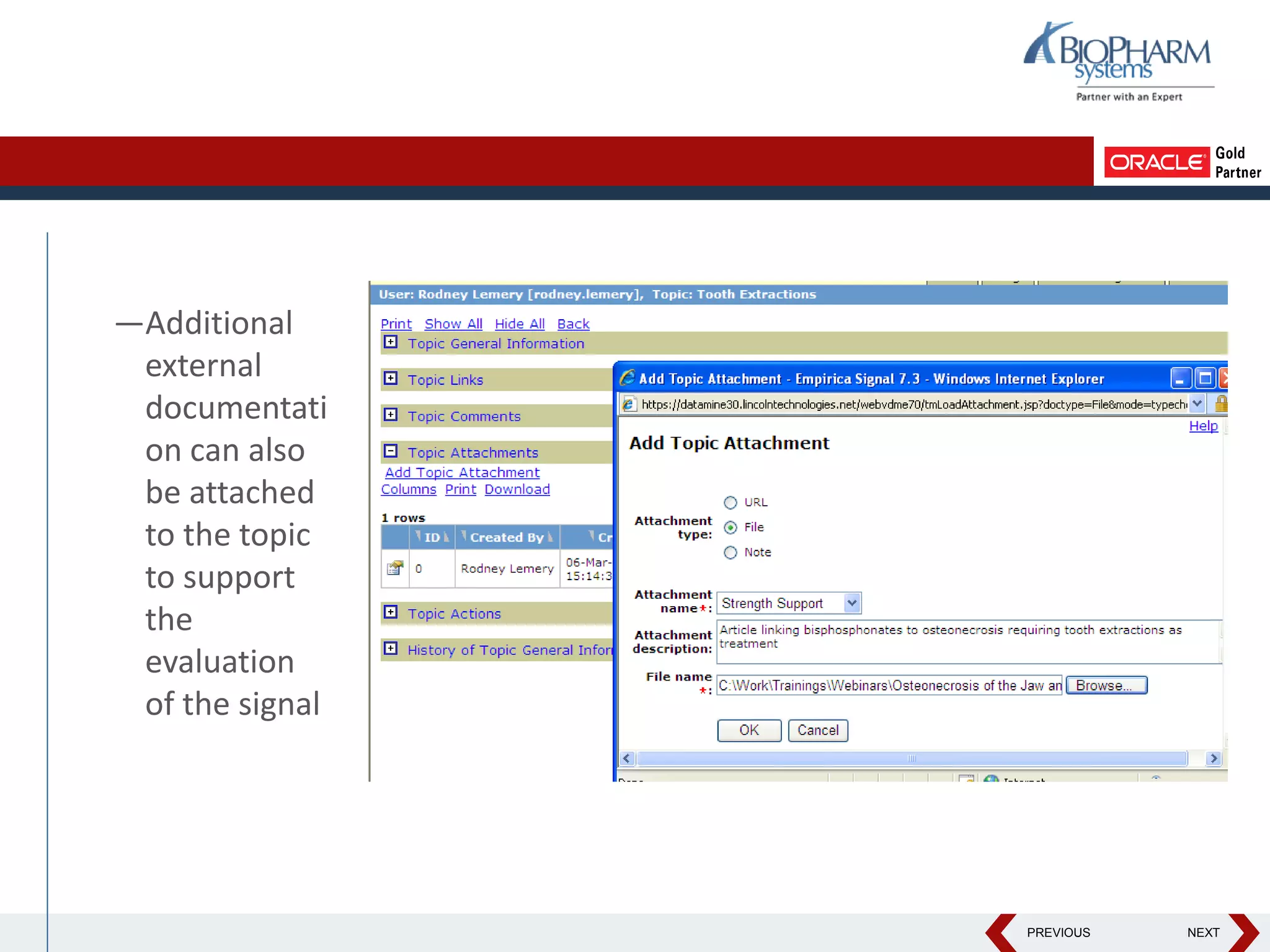
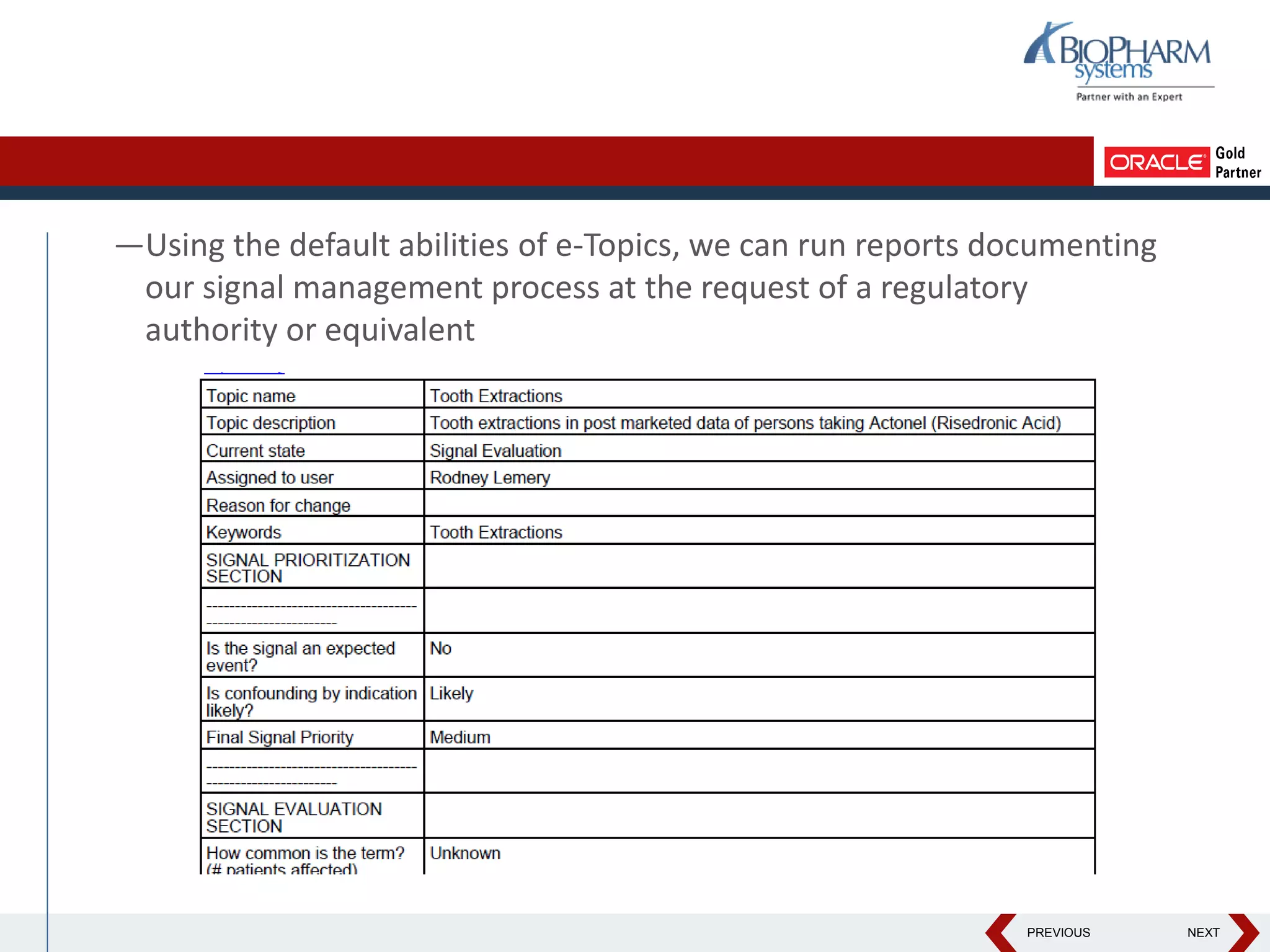
This document discusses using Oracle's Empirica Topics software to document a signal management process. Empirica Topics can provide a systematic workflow for signal management, allowing companies to identify potential issues in data, standardize prioritization and evaluation, and document the entire process for regulatory reporting. The software provides predefined steps for detection, prioritization, evaluation, and reporting of signals. It also allows attachment of case information, documentation, and auditing of the entire signal management process in a single system.