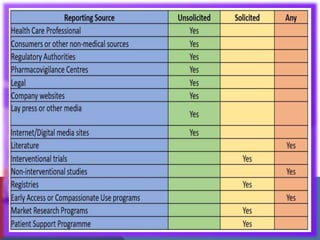
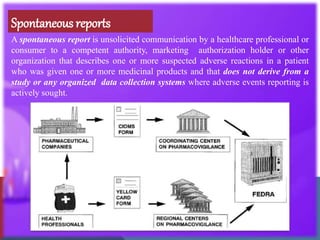
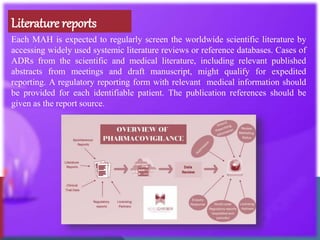
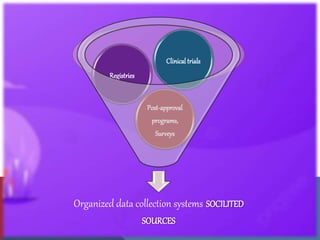
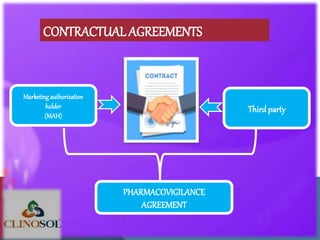
The document outlines the various sources of serious adverse event (SAE) reports in pharmacovigilance, including unsolicited and solicited reports from healthcare professionals, scientific literature, and regulatory authorities. Unsolicited reports arise spontaneously without prior request, while solicited reports are generated from organized data collection systems like clinical trials and patient surveys. It emphasizes the importance of contractual agreements between marketing authorization holders and third parties for the exchange of safety information.