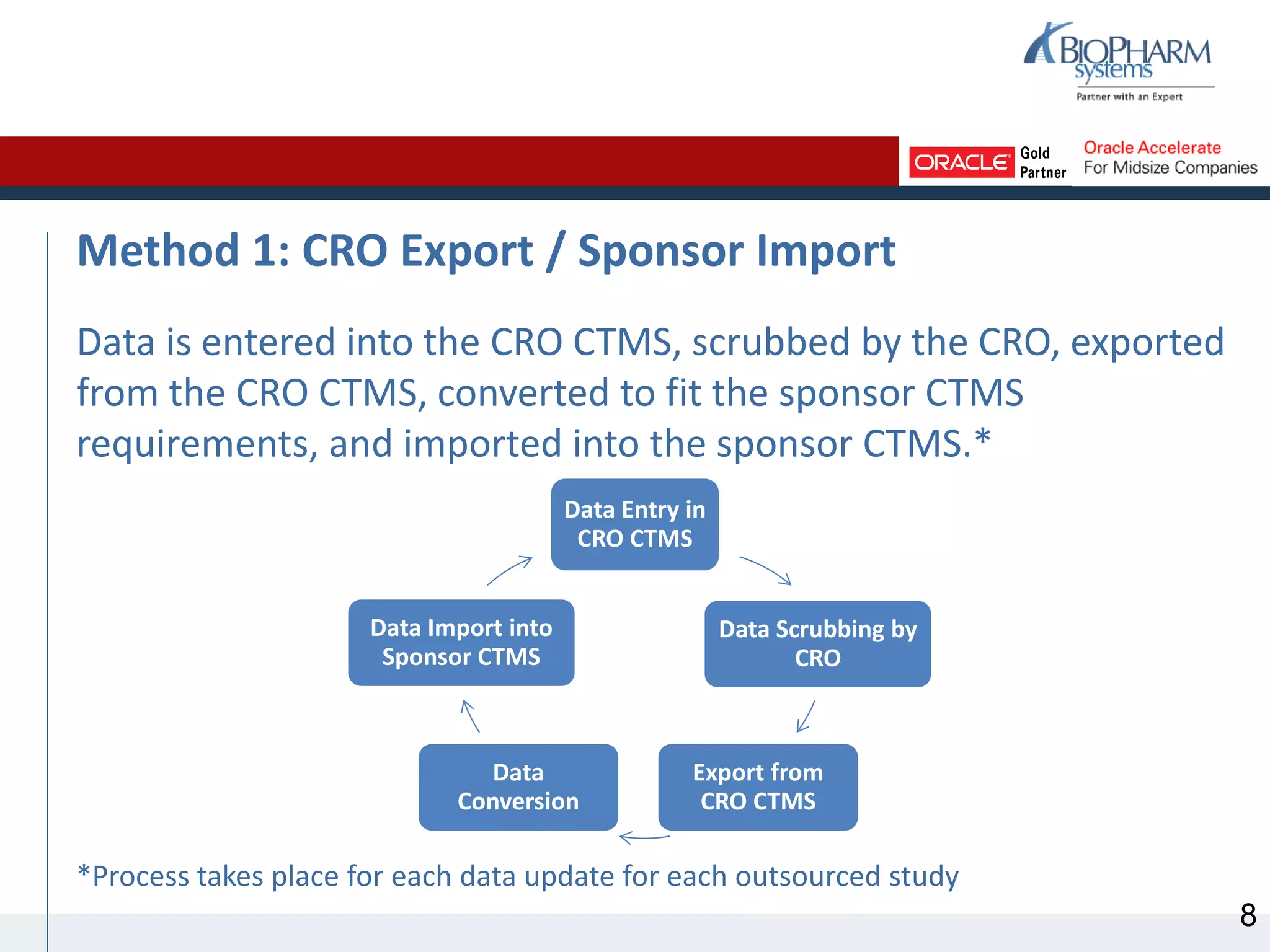
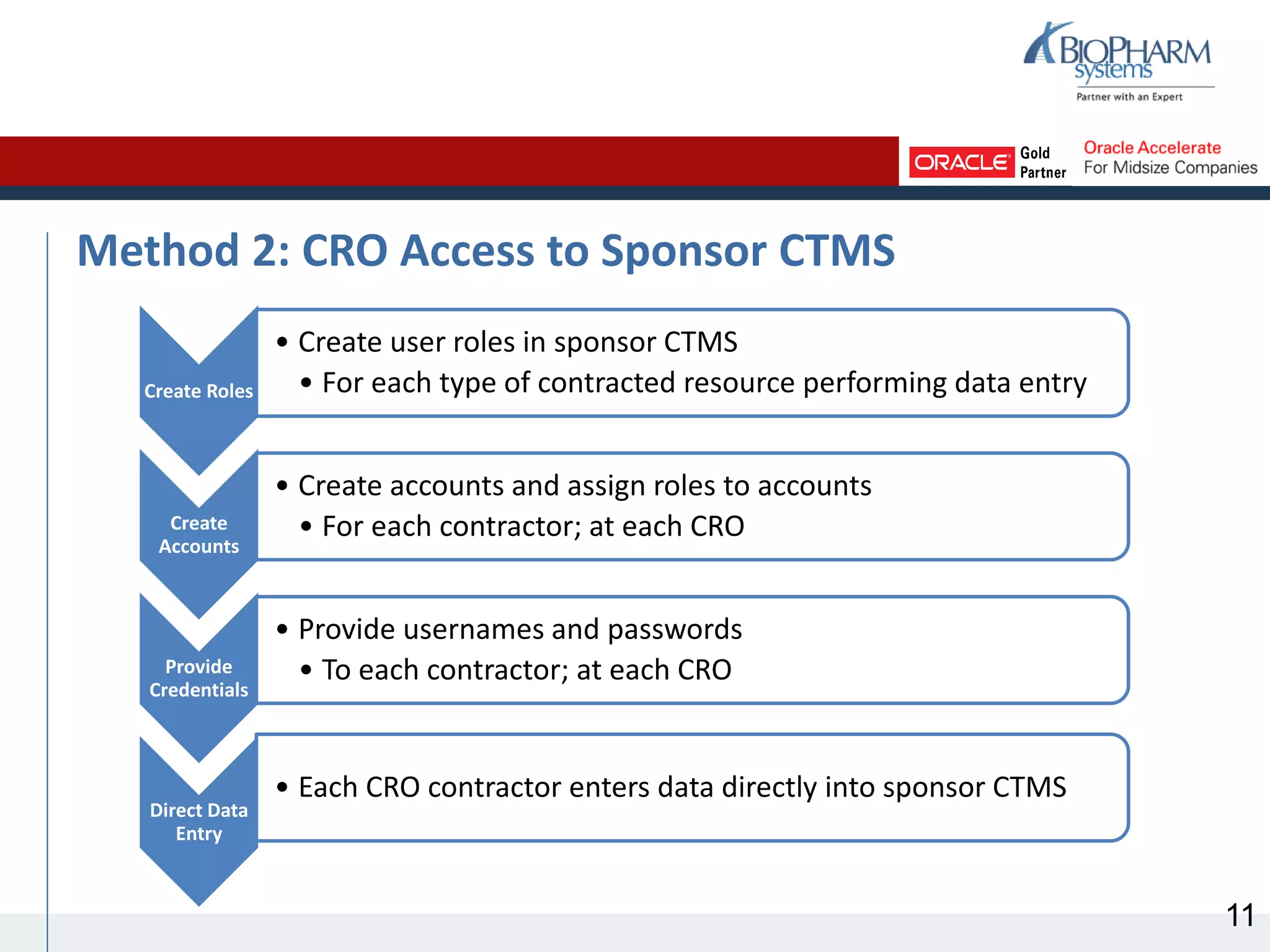
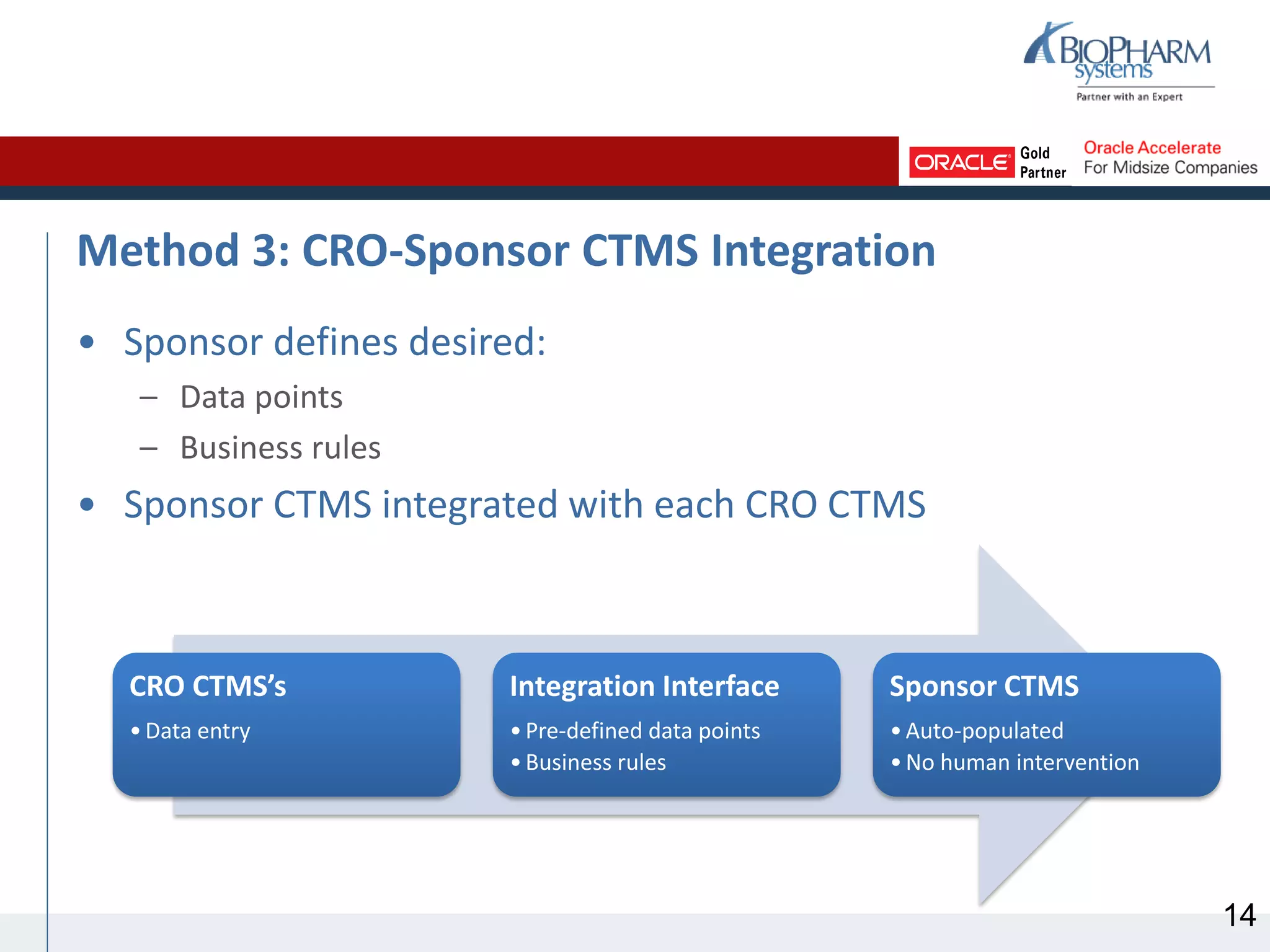
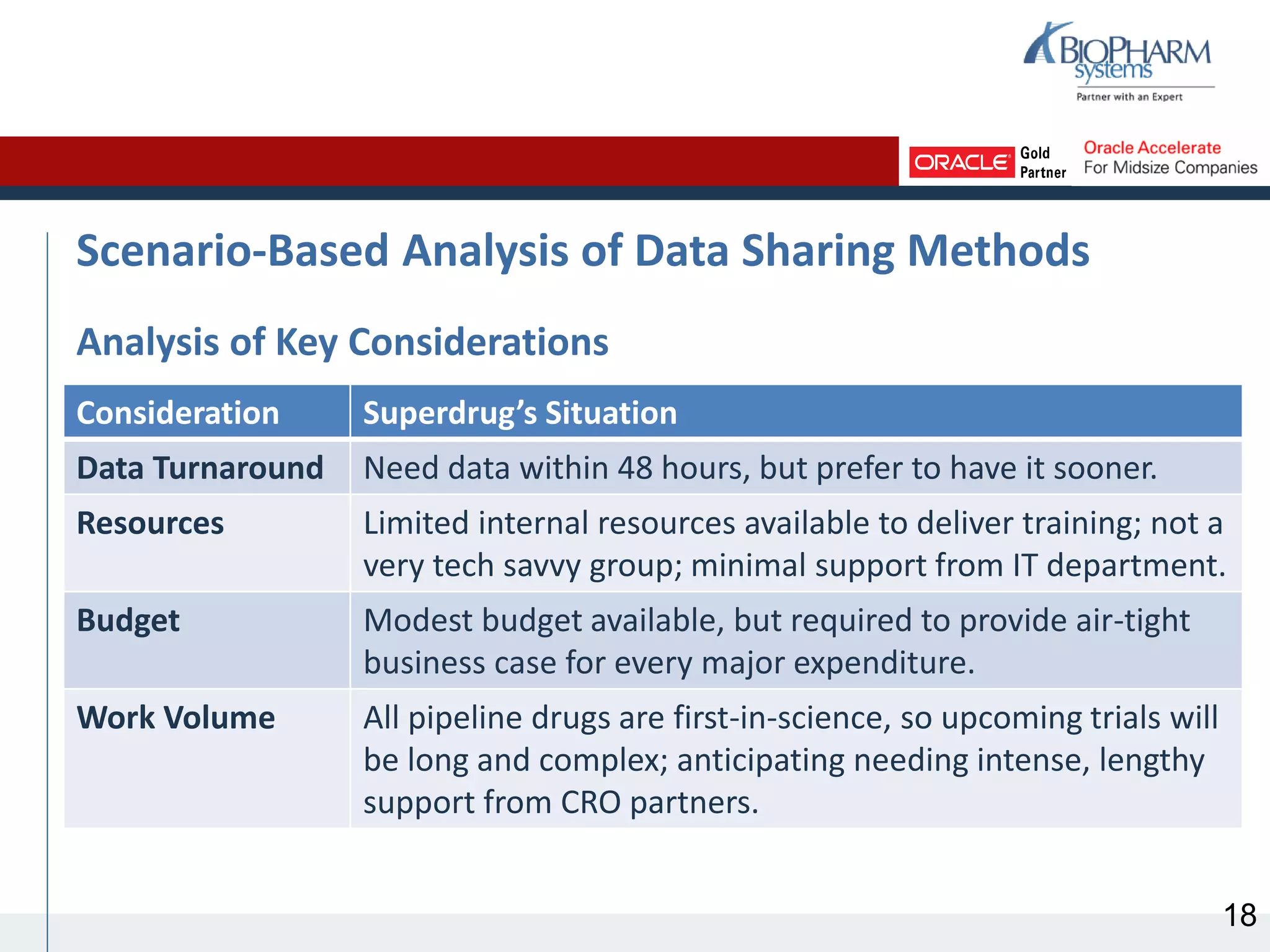
This document discusses different methods for sharing clinical trial management system (CTMS) data between sponsors and contract research organizations (CROs). It begins with an overview of current industry trends driving increased outsourcing to CROs and the need for collaboration on data sharing. Three main methods are described: CRO export/sponsor import, CRO access to sponsor CTMS, and CRO-sponsor CTMS integration. For each, benefits and drawbacks are outlined. A scenario analysis examines applying the methods to a fictional sponsor's specific needs and resources. The analysis recommends CRO-sponsor integration as the best option to meet the sponsor's requirements for timely data, limited resources, and scalability to support growth.