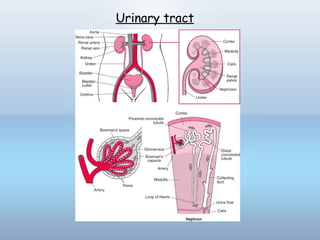
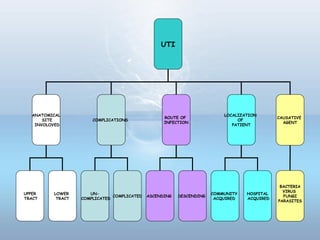
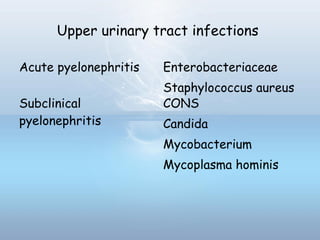
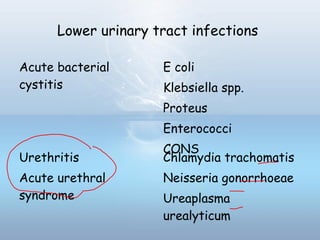
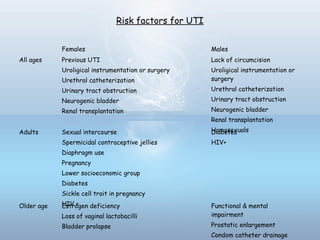
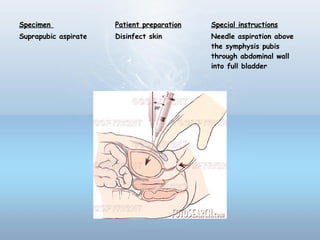
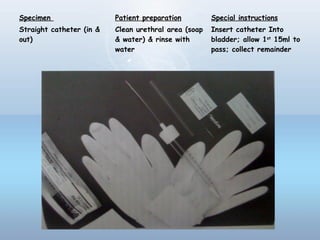
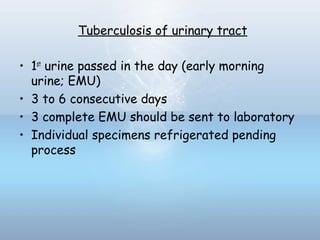
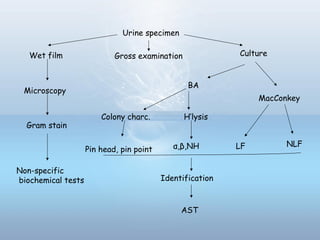
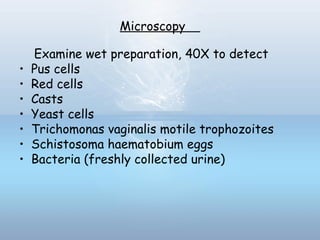
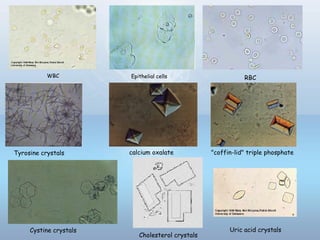
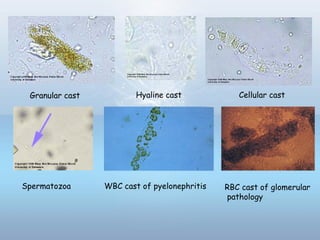
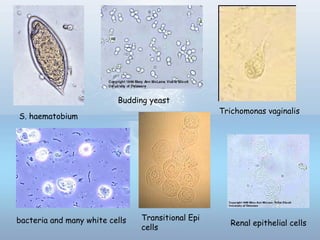
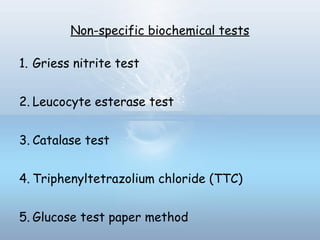
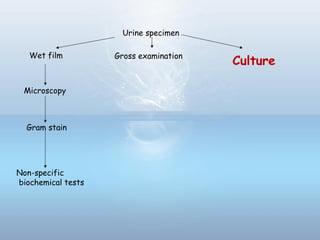
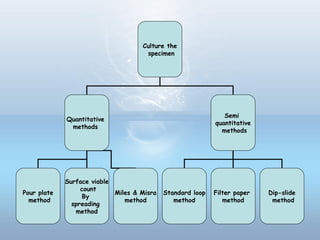
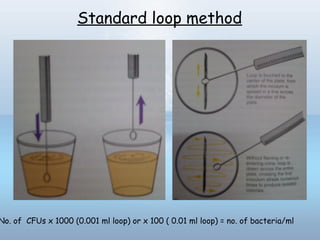
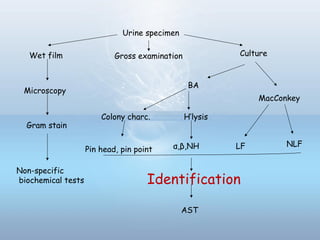
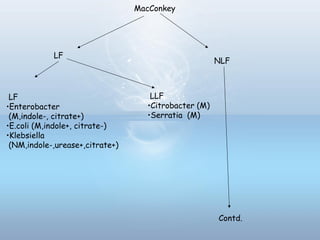
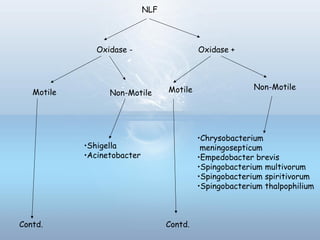
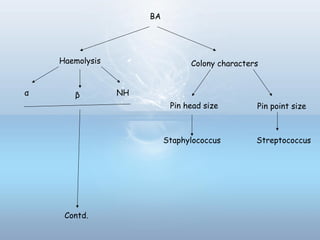
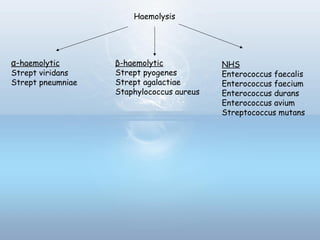
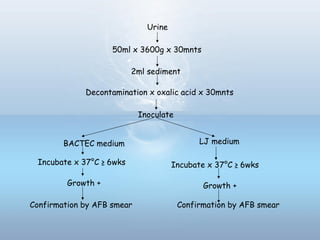
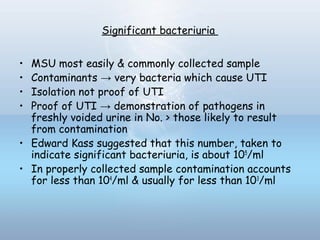
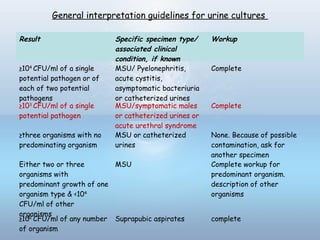
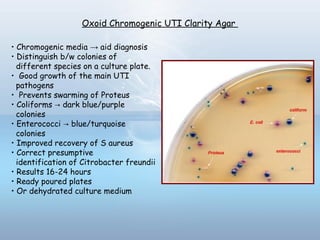
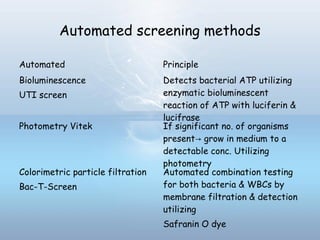
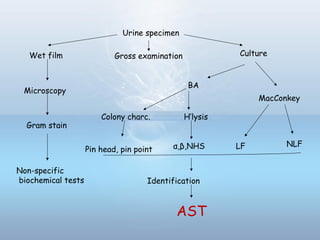
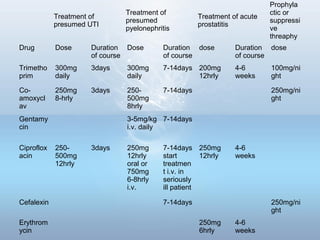
This document provides information on urinary tract infections (UTIs), including their definition, anatomical structures involved, classification, etiology, pathogenesis, signs and symptoms, specimen collection and transport, laboratory diagnosis and interpretation, antimicrobial susceptibility testing, treatment, and references. UTIs are caused by microbial invasion of the genitourinary tract and are extremely common. Proper collection and testing of urine specimens is required for accurate laboratory diagnosis and interpretation of results to determine treatment.