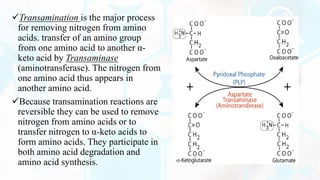
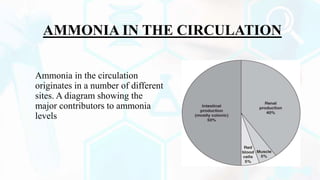
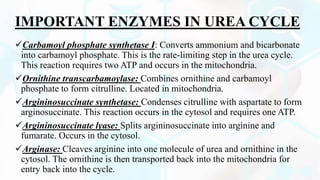
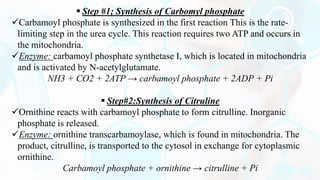
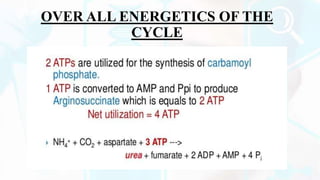
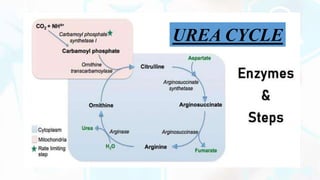
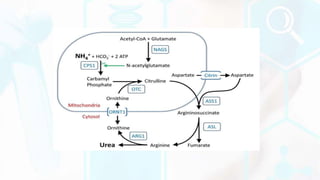
The document summarizes the metabolism of proteins and amino acids, focusing on the urea cycle. It describes how amino acids are broken down to remove nitrogen, producing ammonia. Ammonia is highly toxic, so the liver uses the urea cycle to convert ammonia to urea for excretion. The urea cycle involves 5 enzymatic reactions that occur in the liver mitochondria and cytosol. Defects in urea cycle enzymes can cause hyperammonemia, which can be fatal if not treated.