Acid base maintenance.
Biochemistry
Acid base maintenance
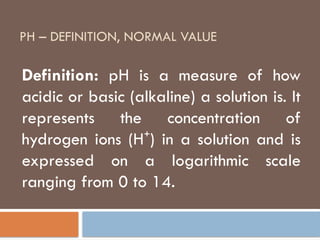
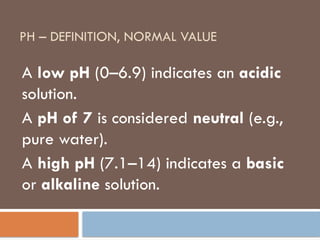
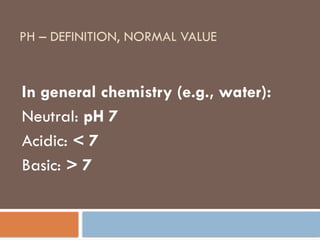
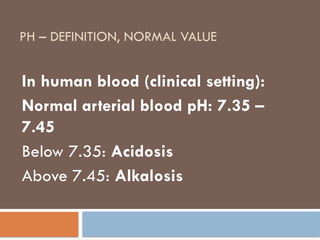
pH – definition, normal value
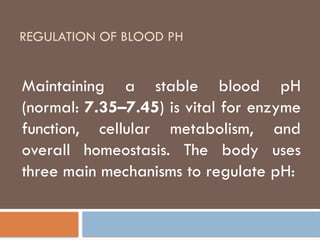
Regulation of blood PH
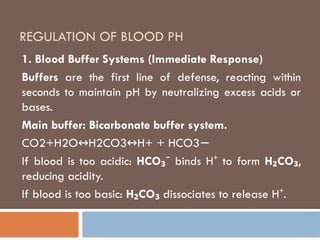
Blood buffer system
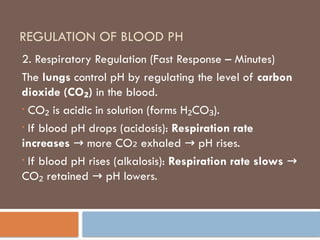
Respiratory regulation
Renal regulation
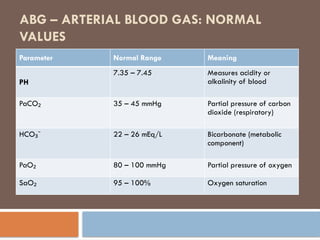
Arterial Blood Gas
ABG Normal Value
Acid base disorder
types of acid base
definition of acid base
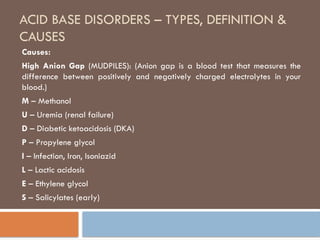
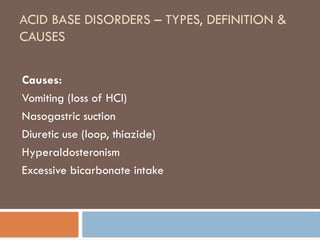
causes of acid base
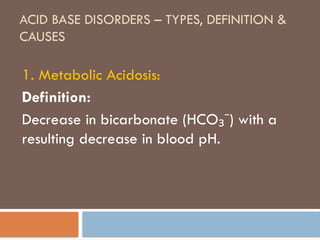
Metabolic acidosis
Metabolic alkalosis
Respiratory acidosis
Respiratory alkalosis
Hyperventilation
Nursing
Nutrition