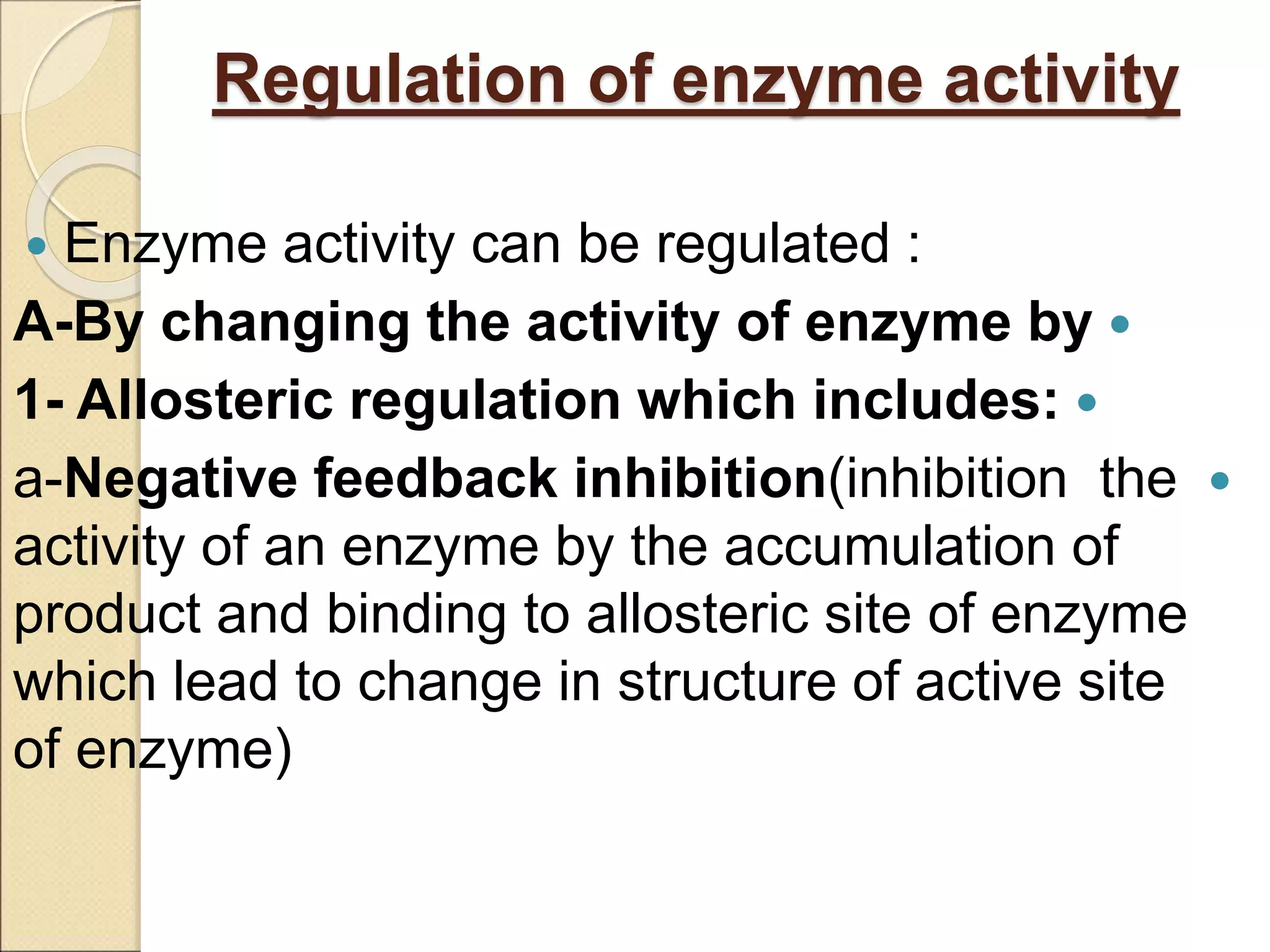
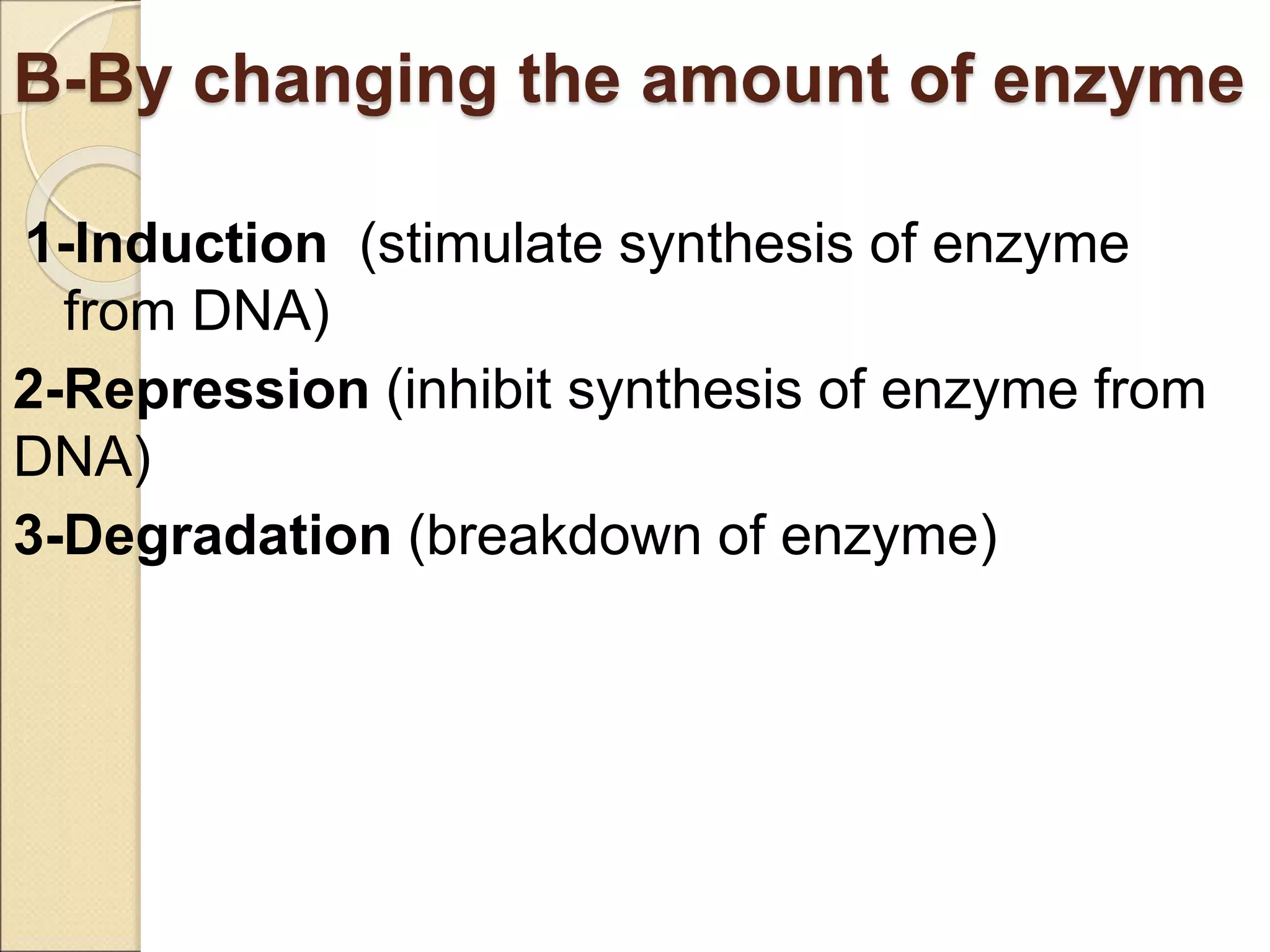
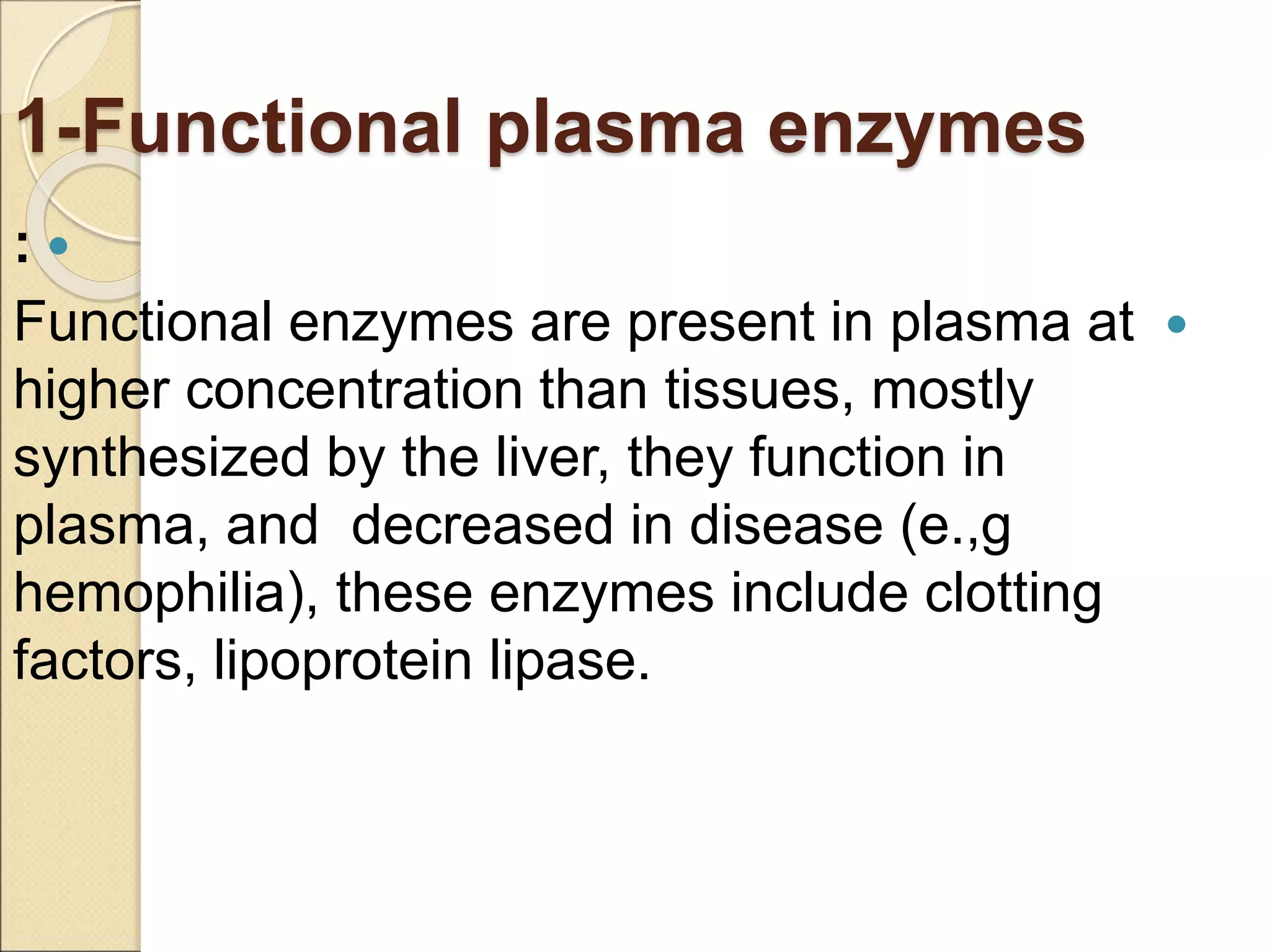
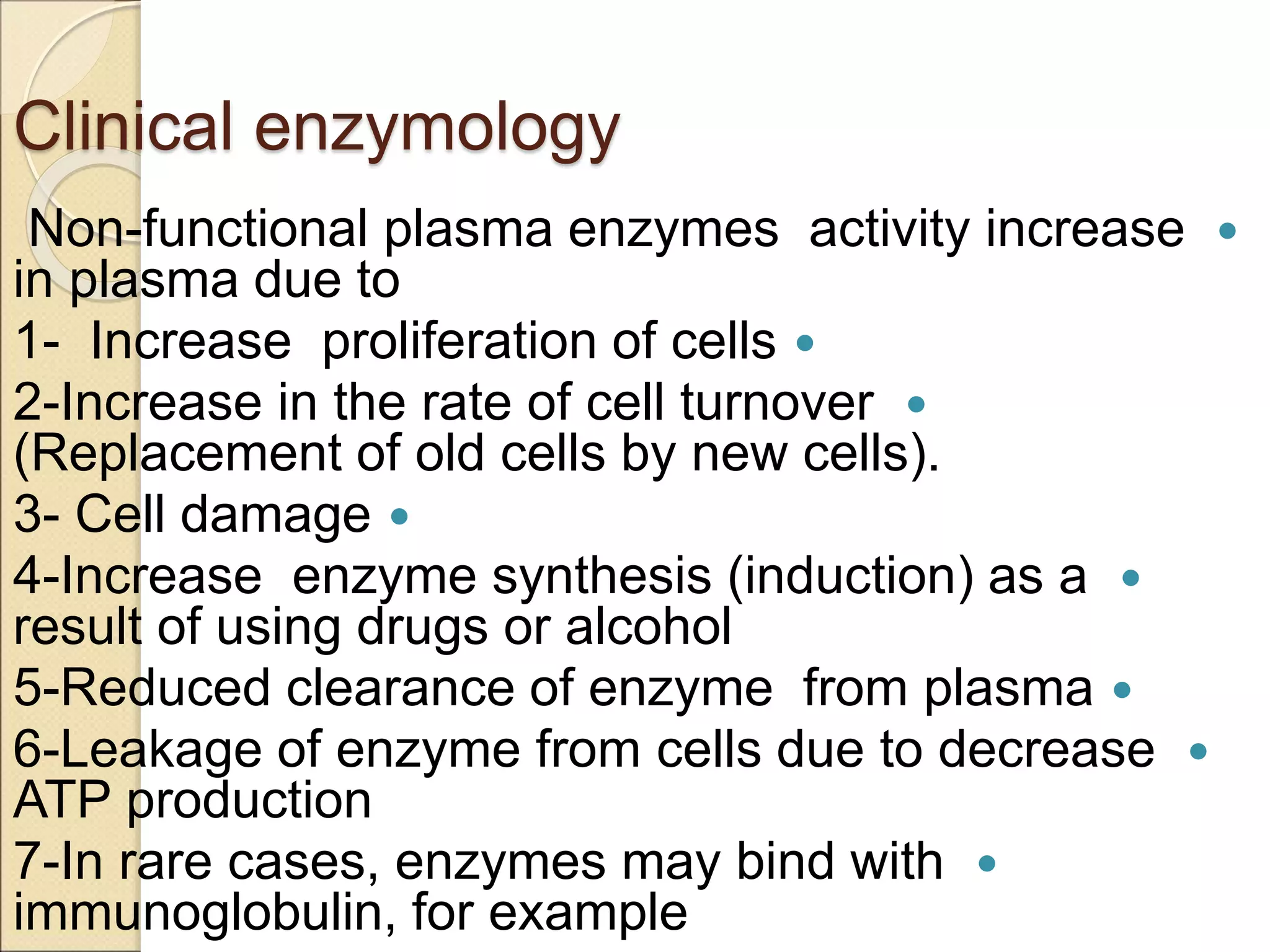
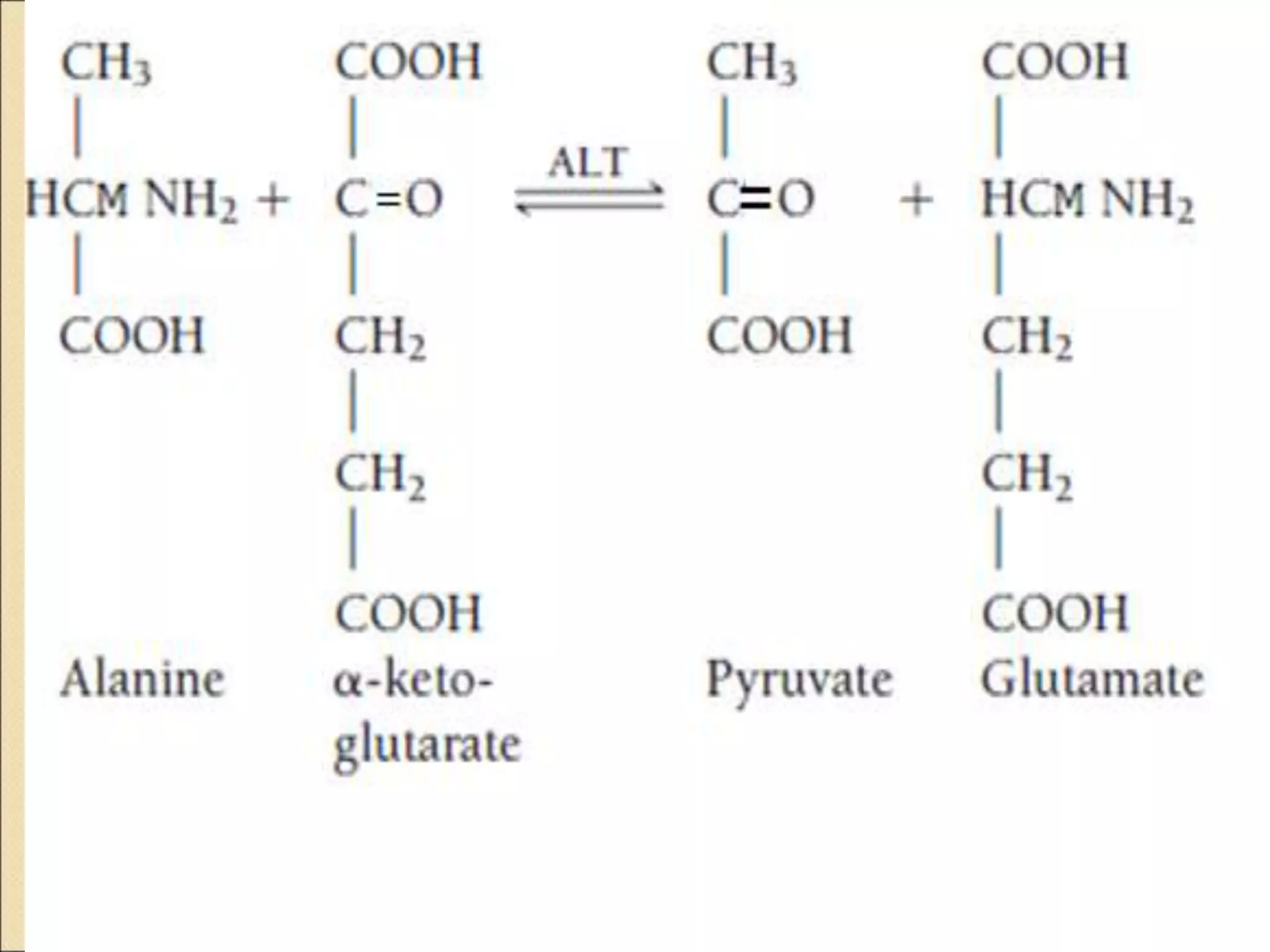
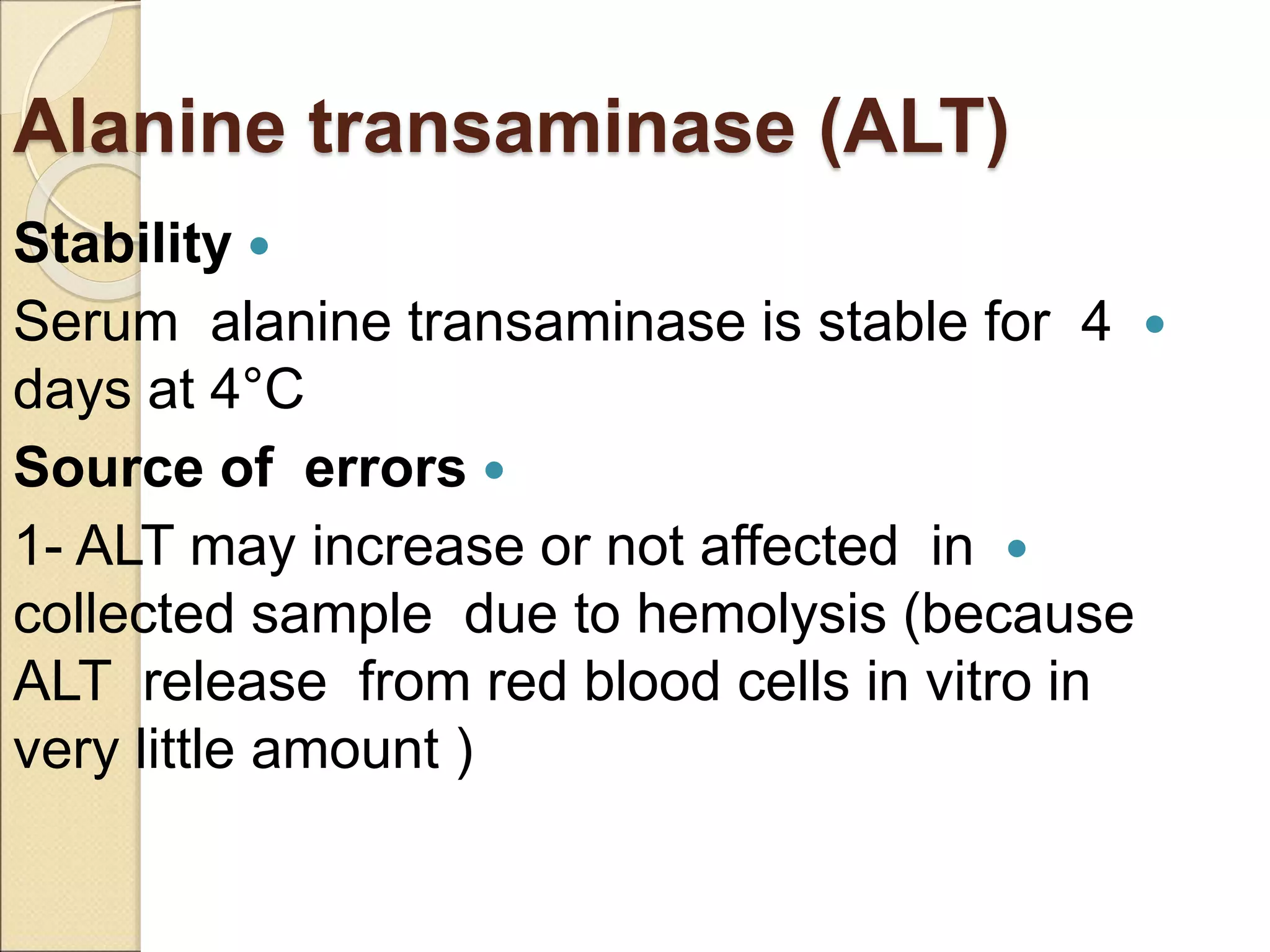
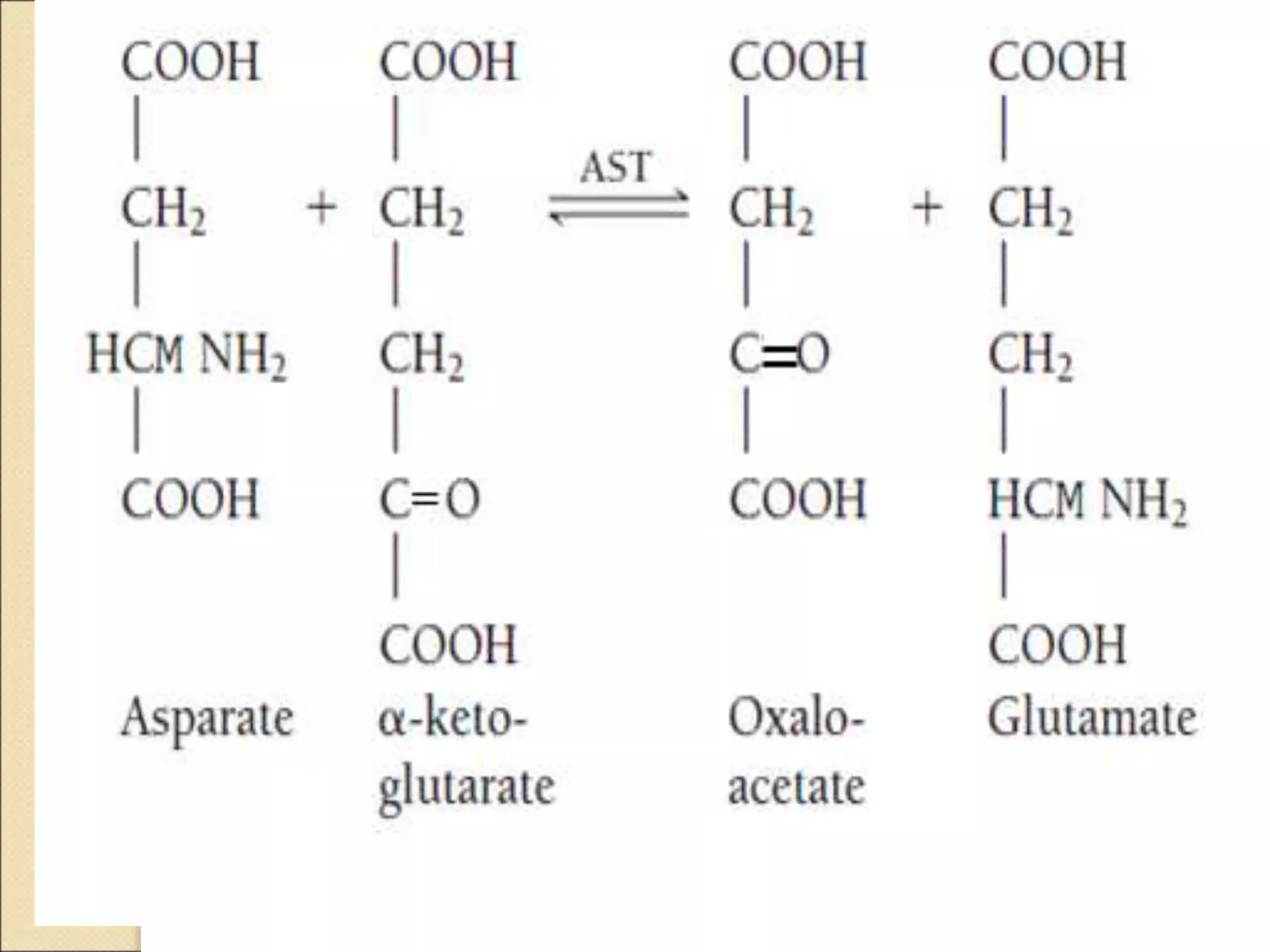
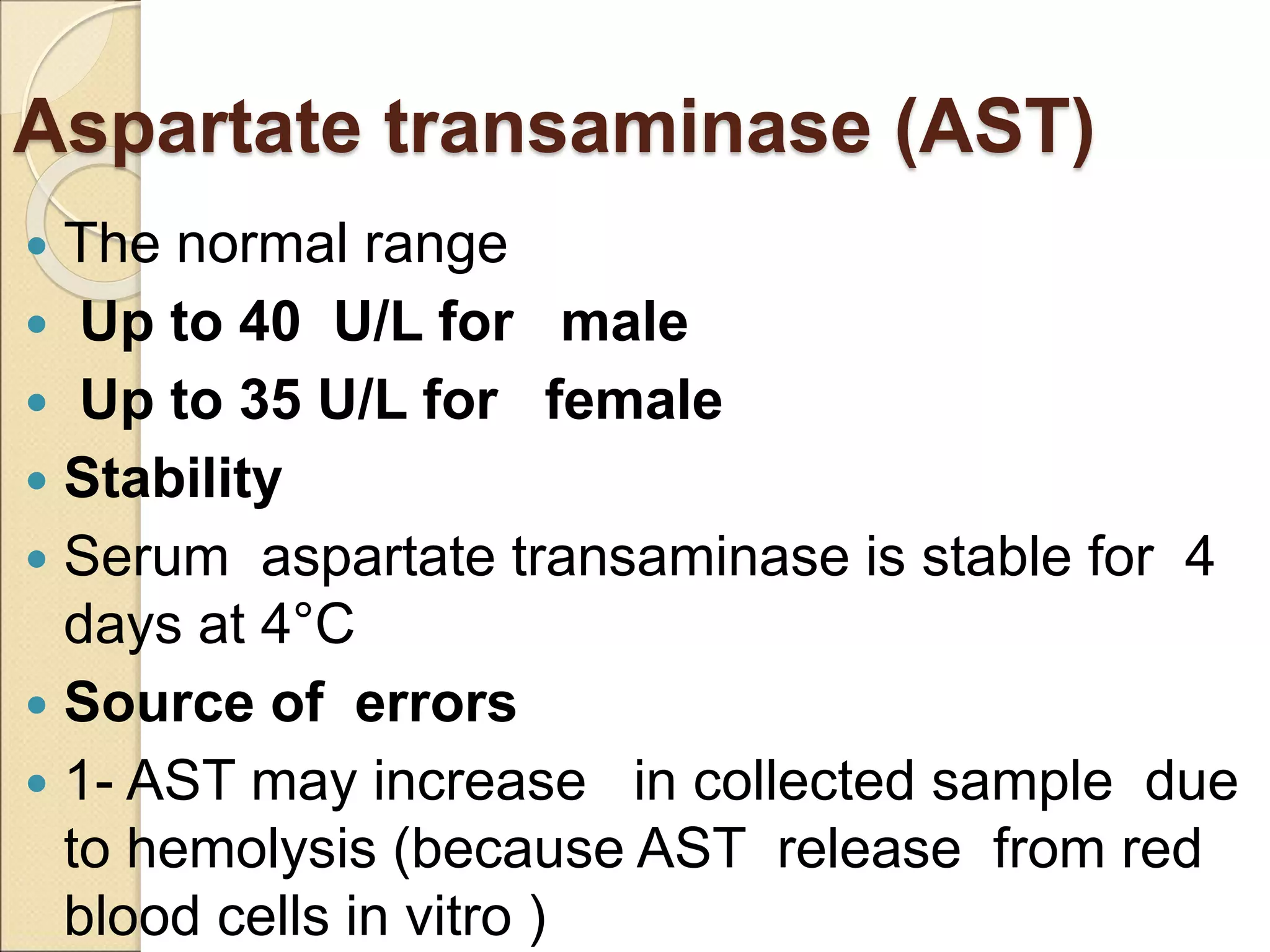
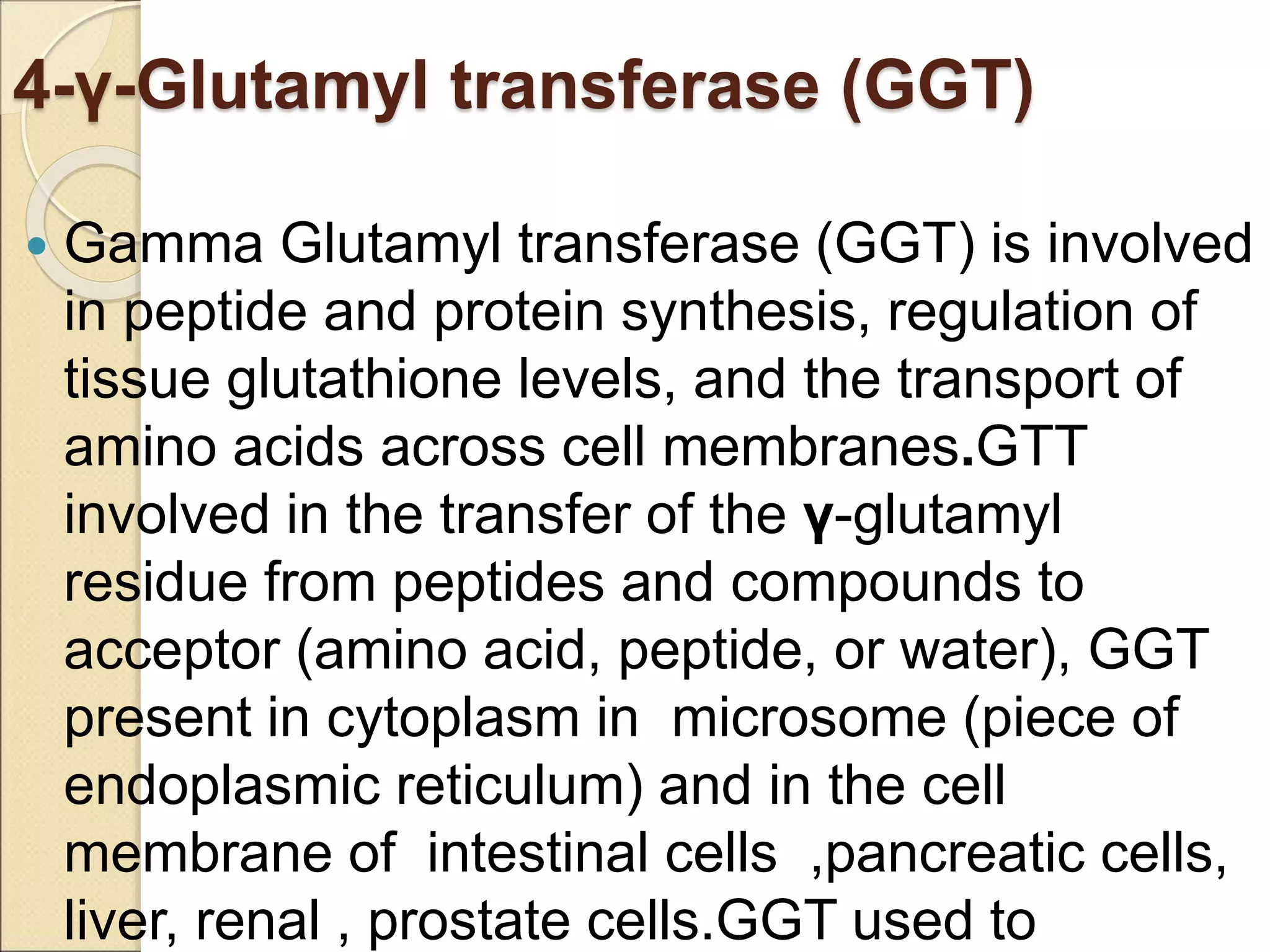
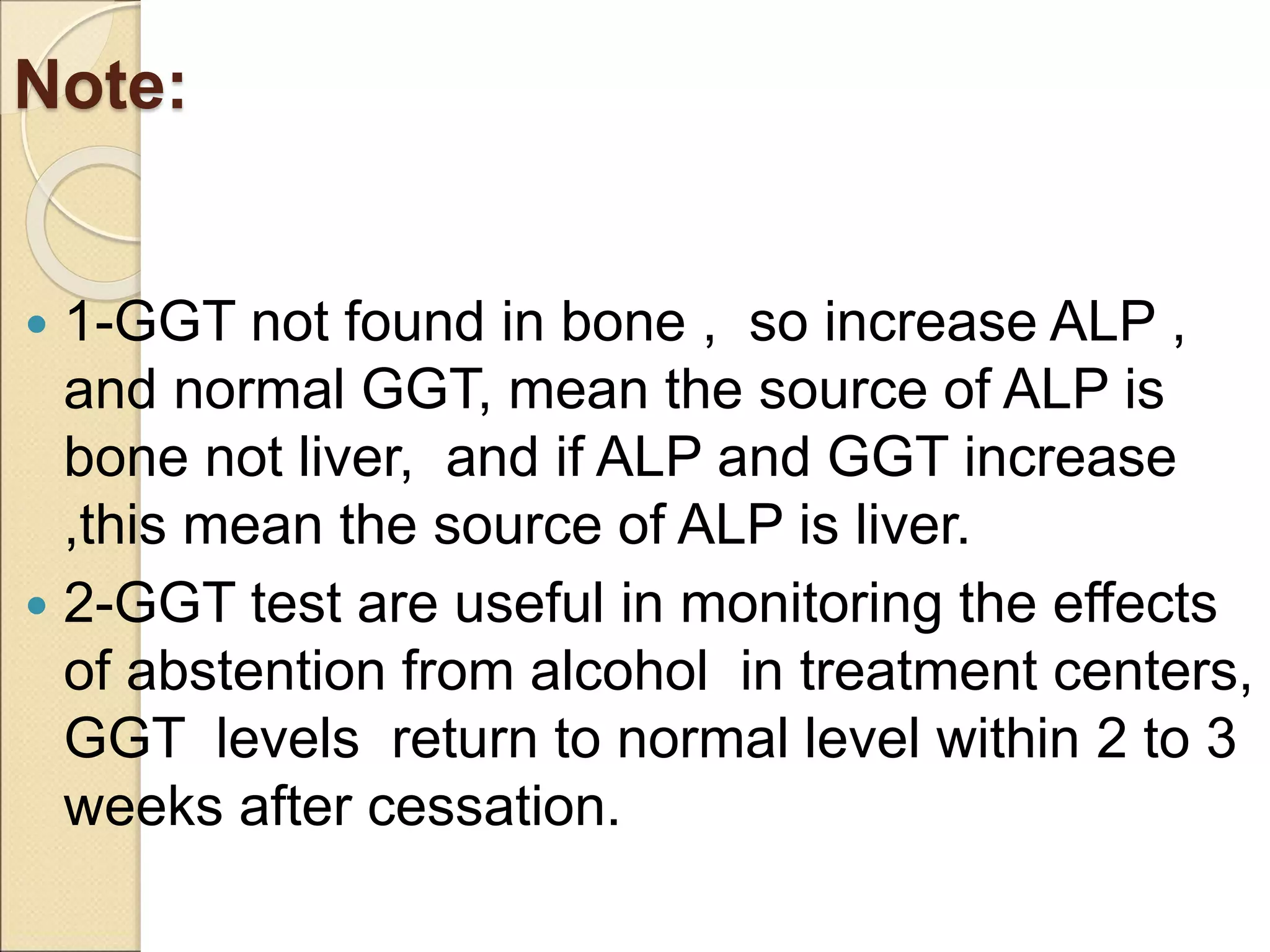
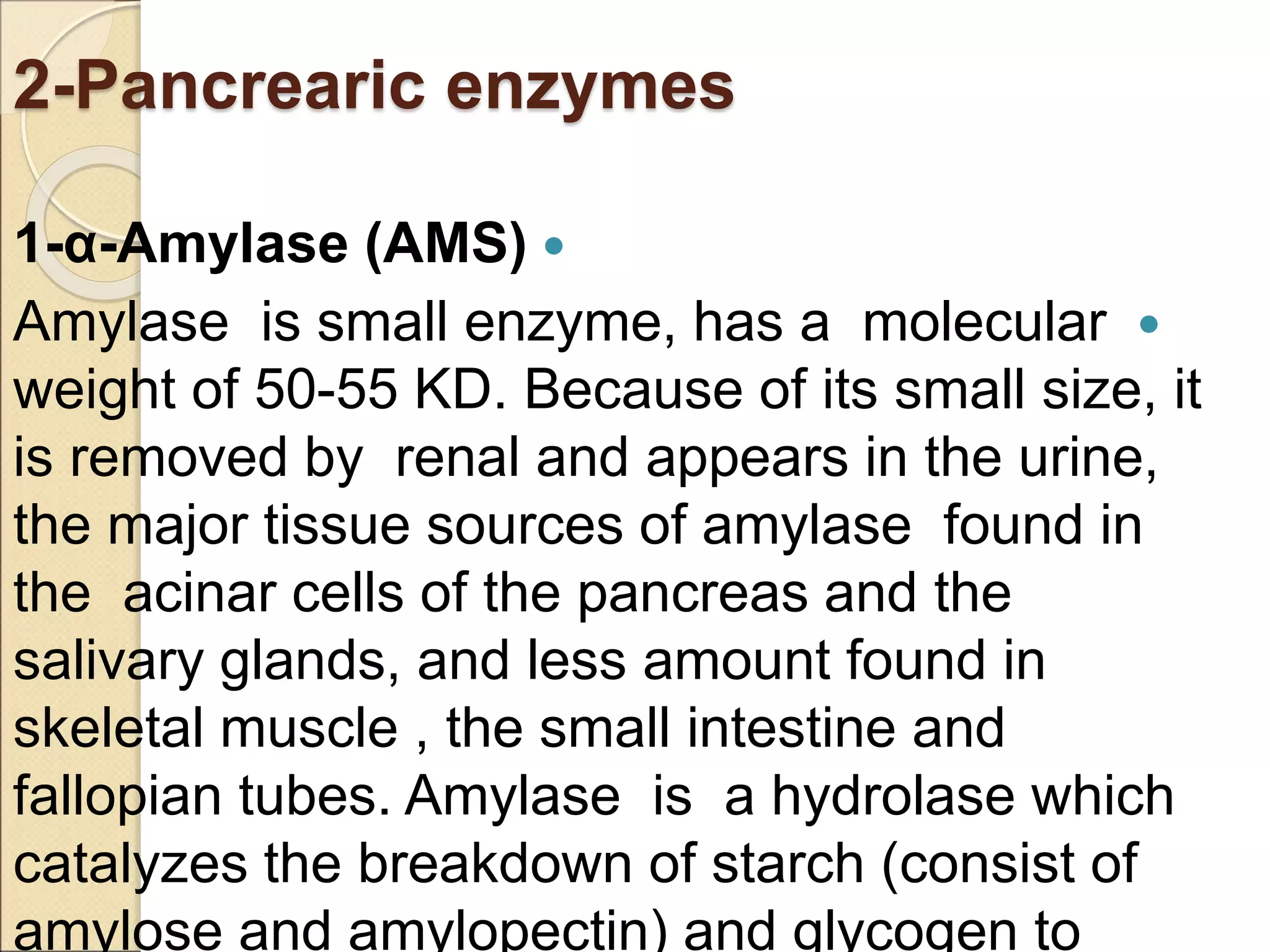
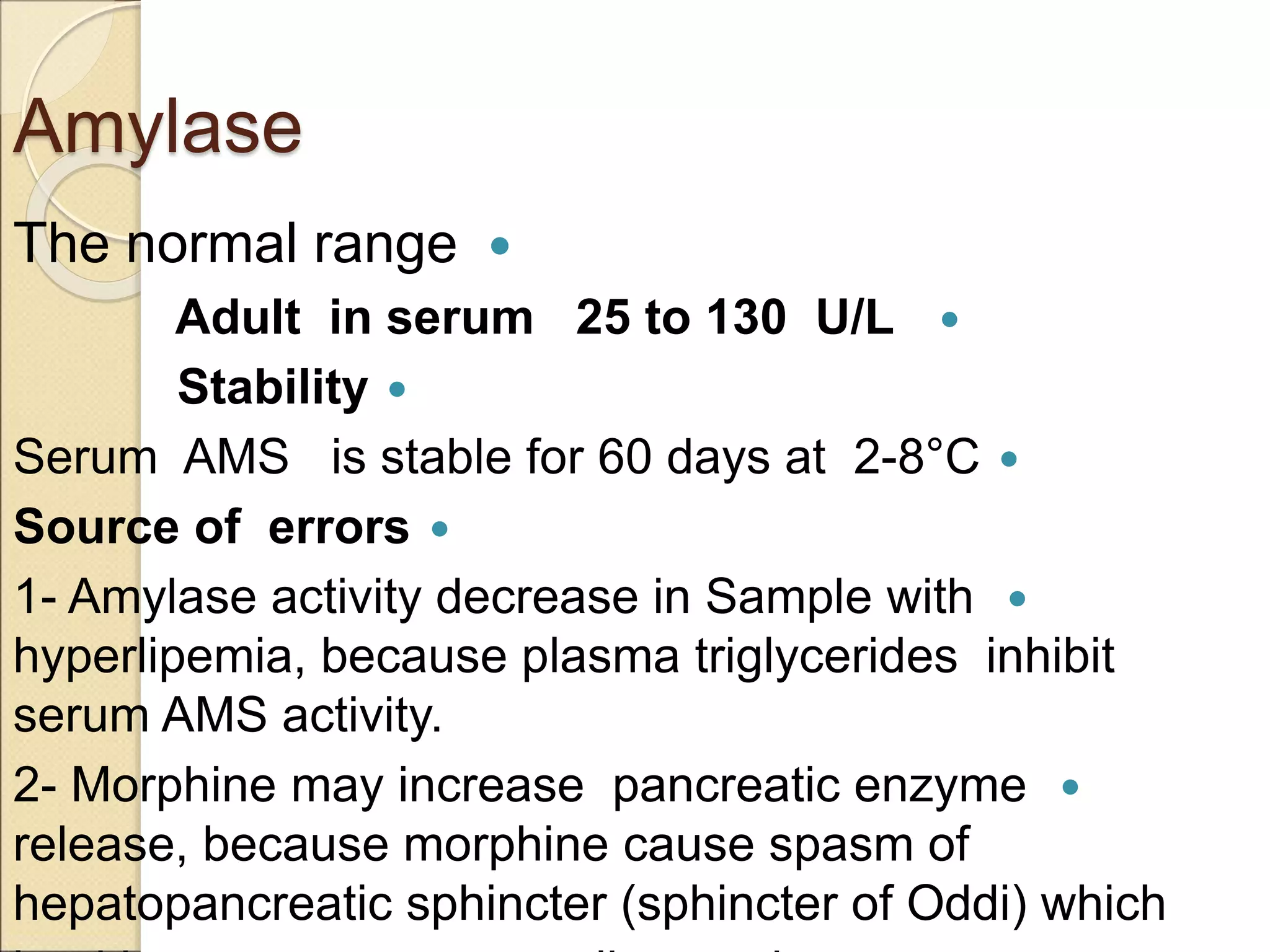
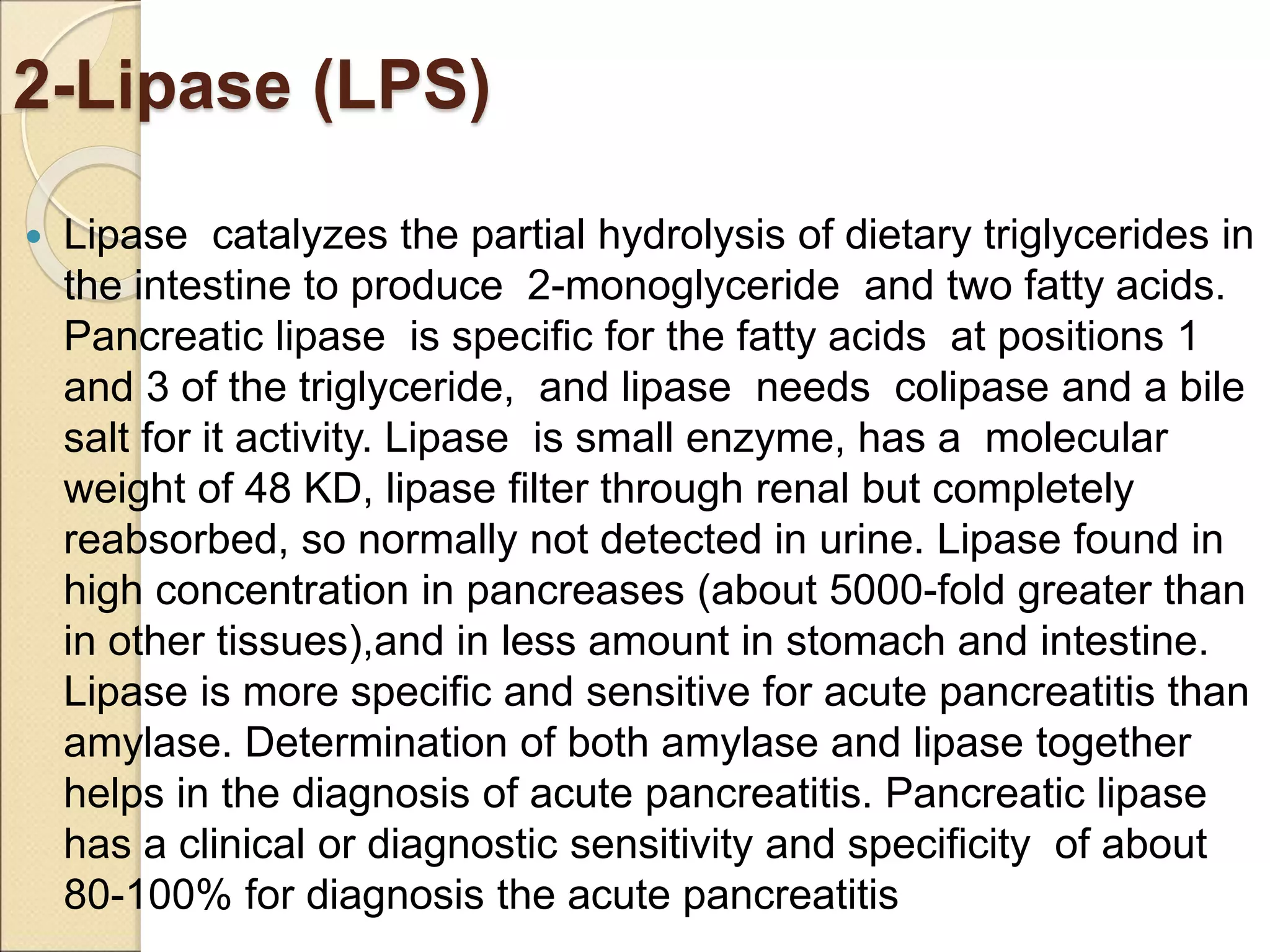
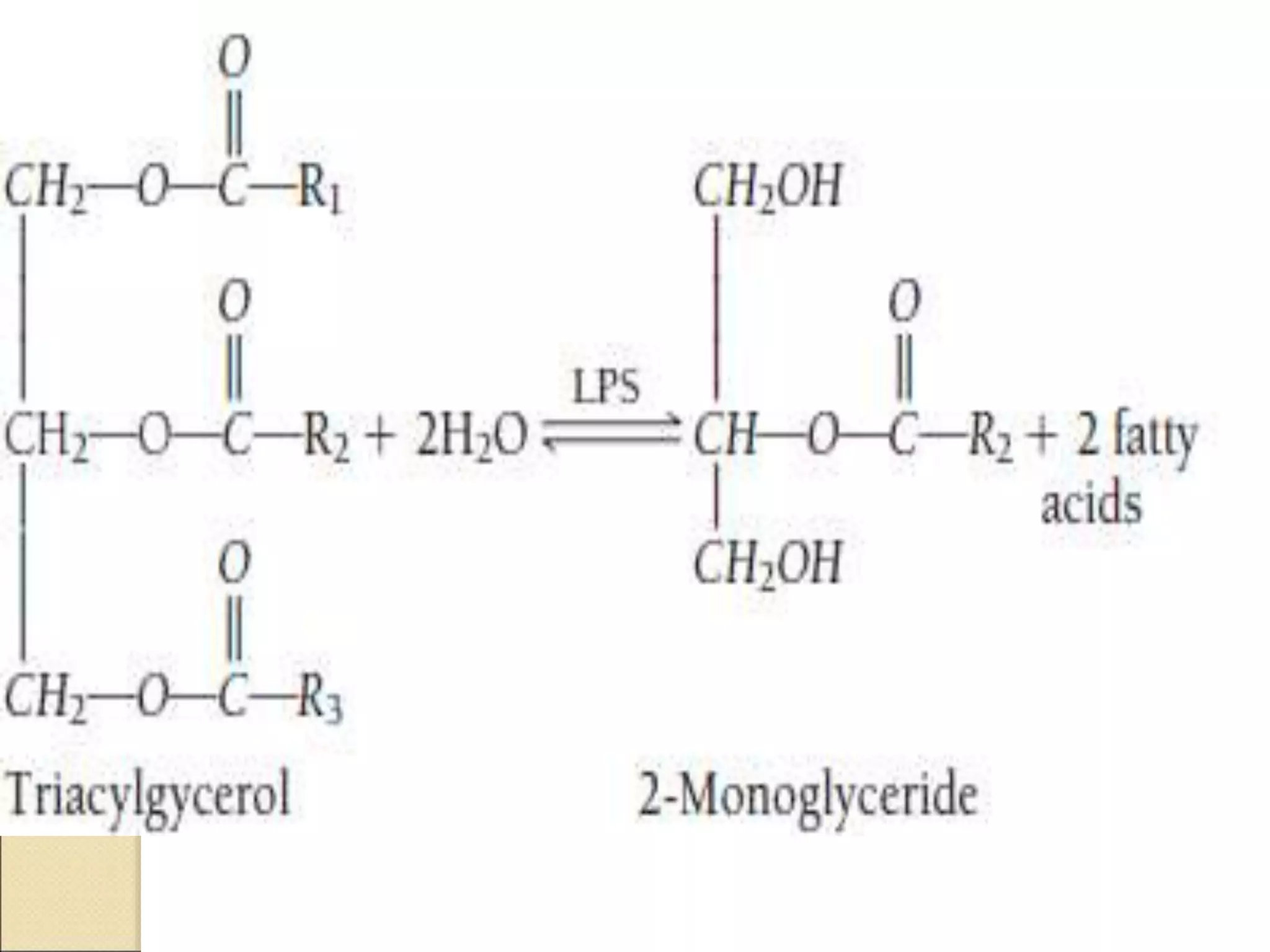
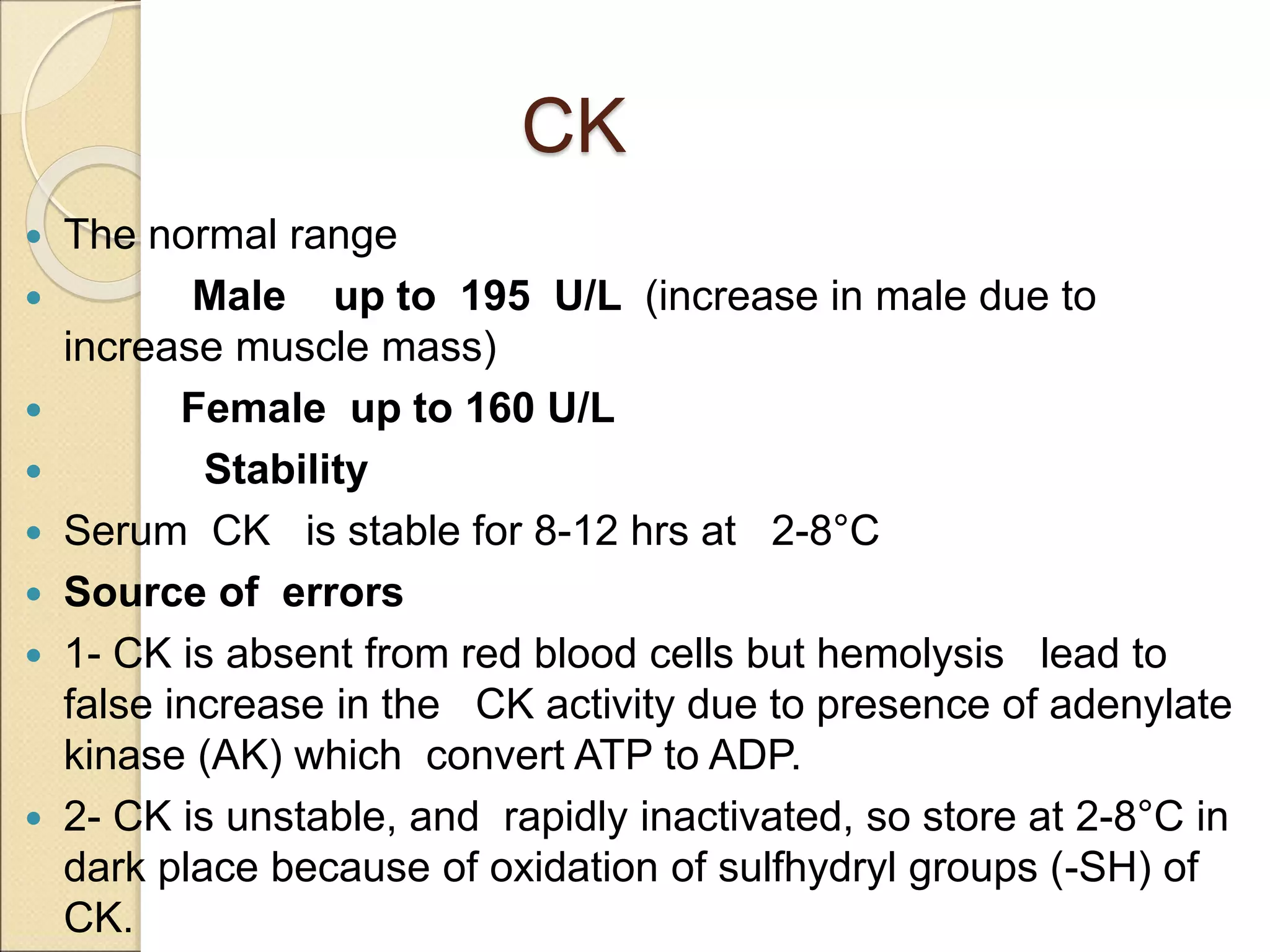
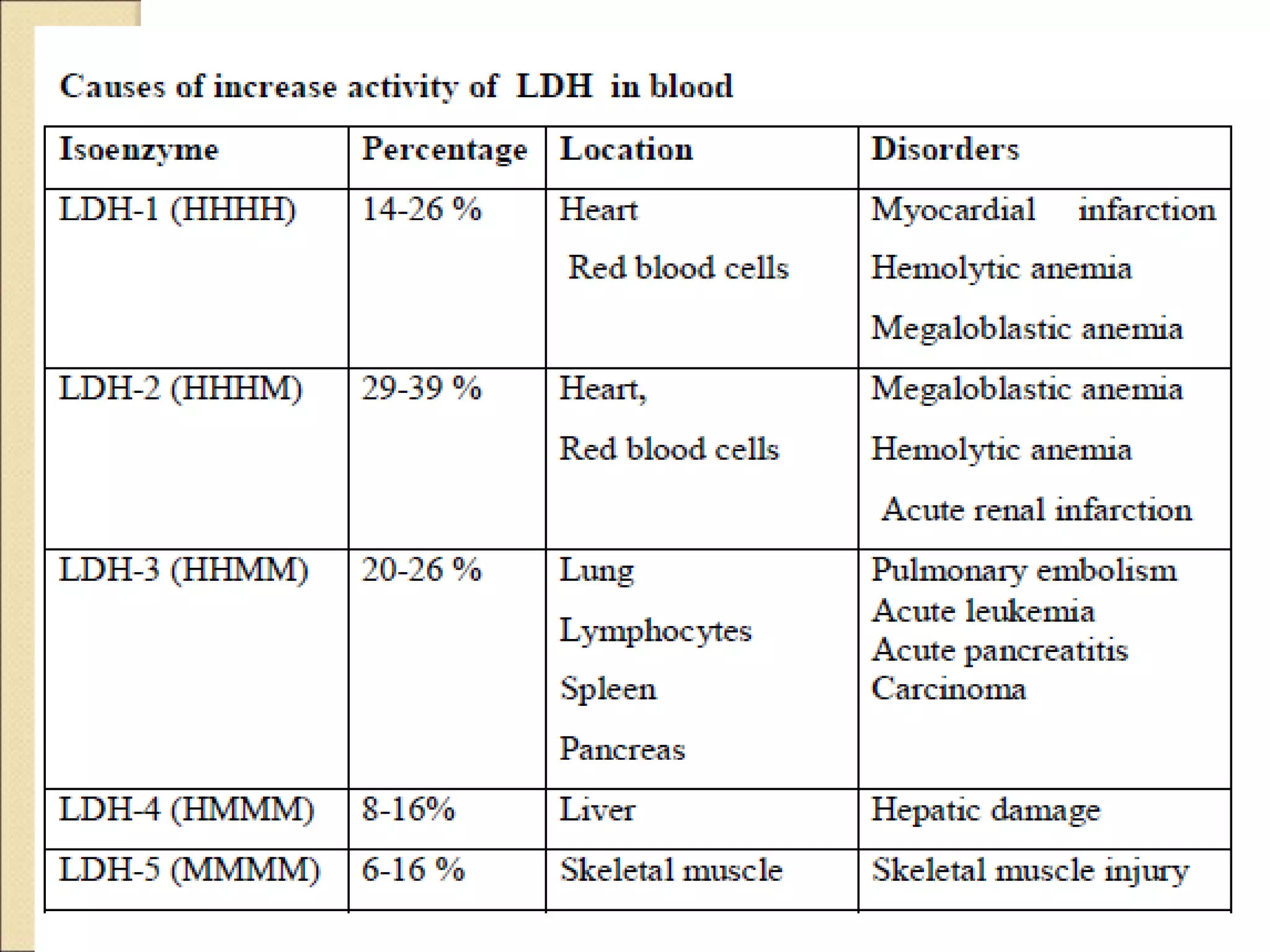
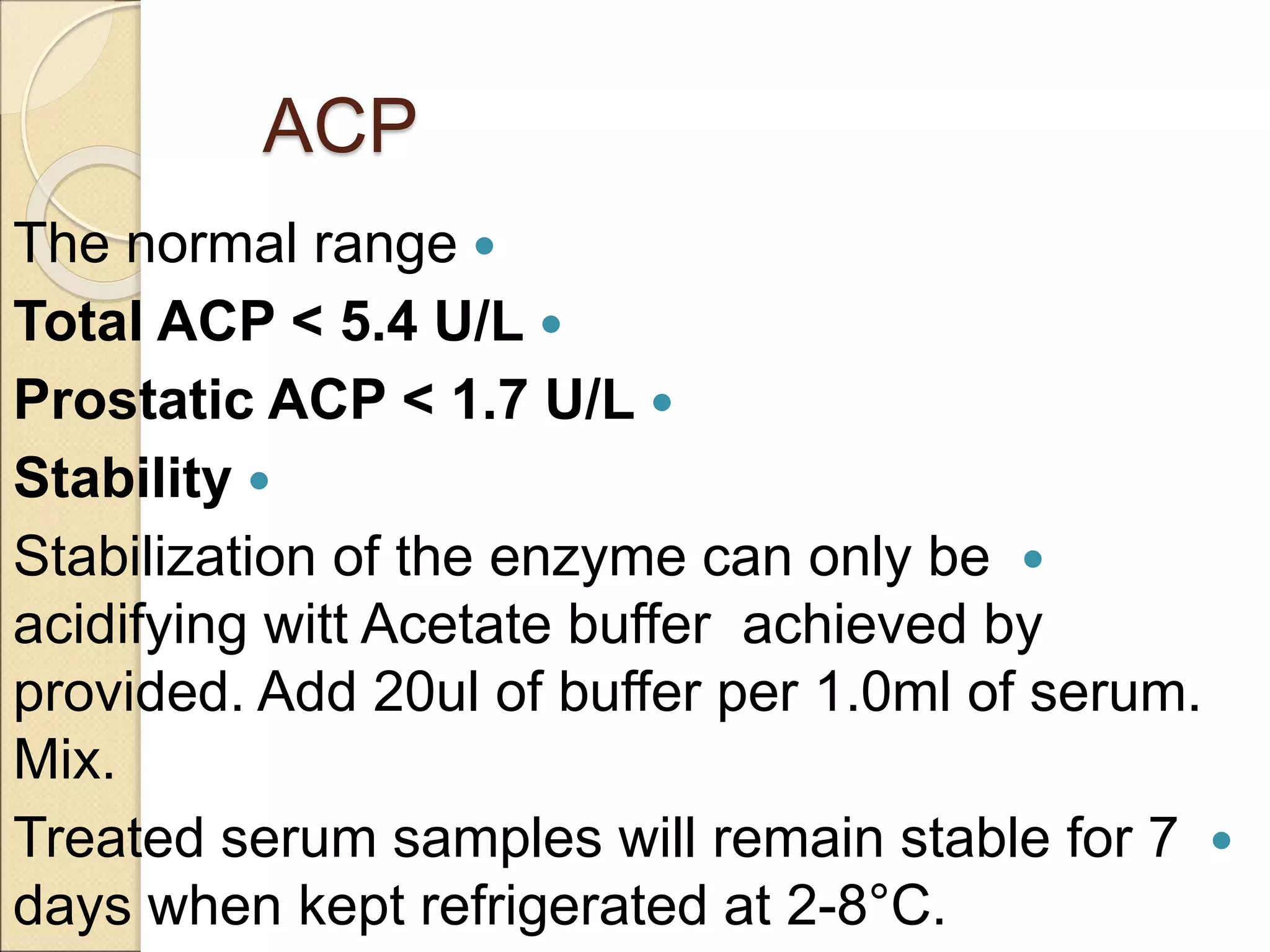
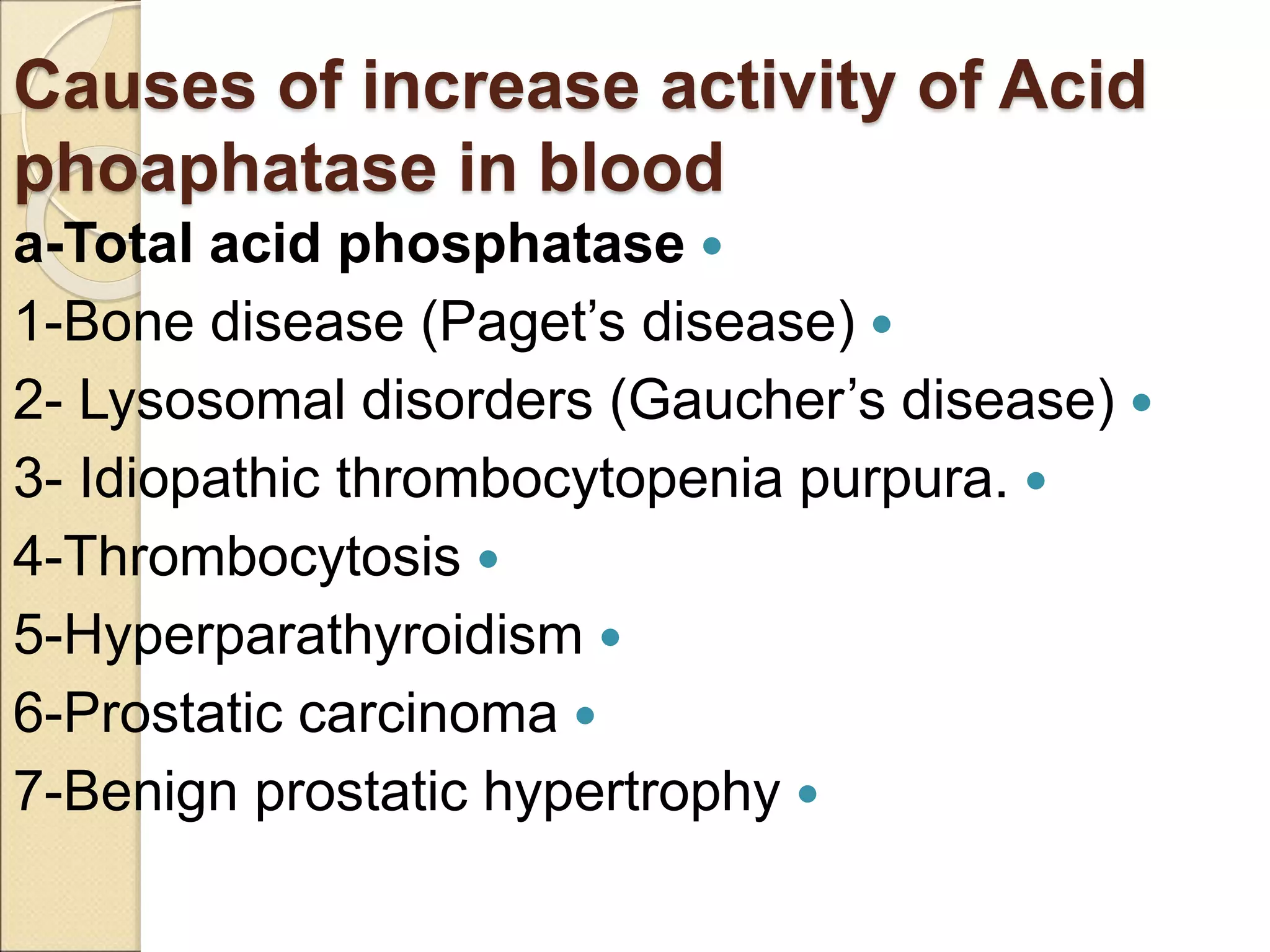
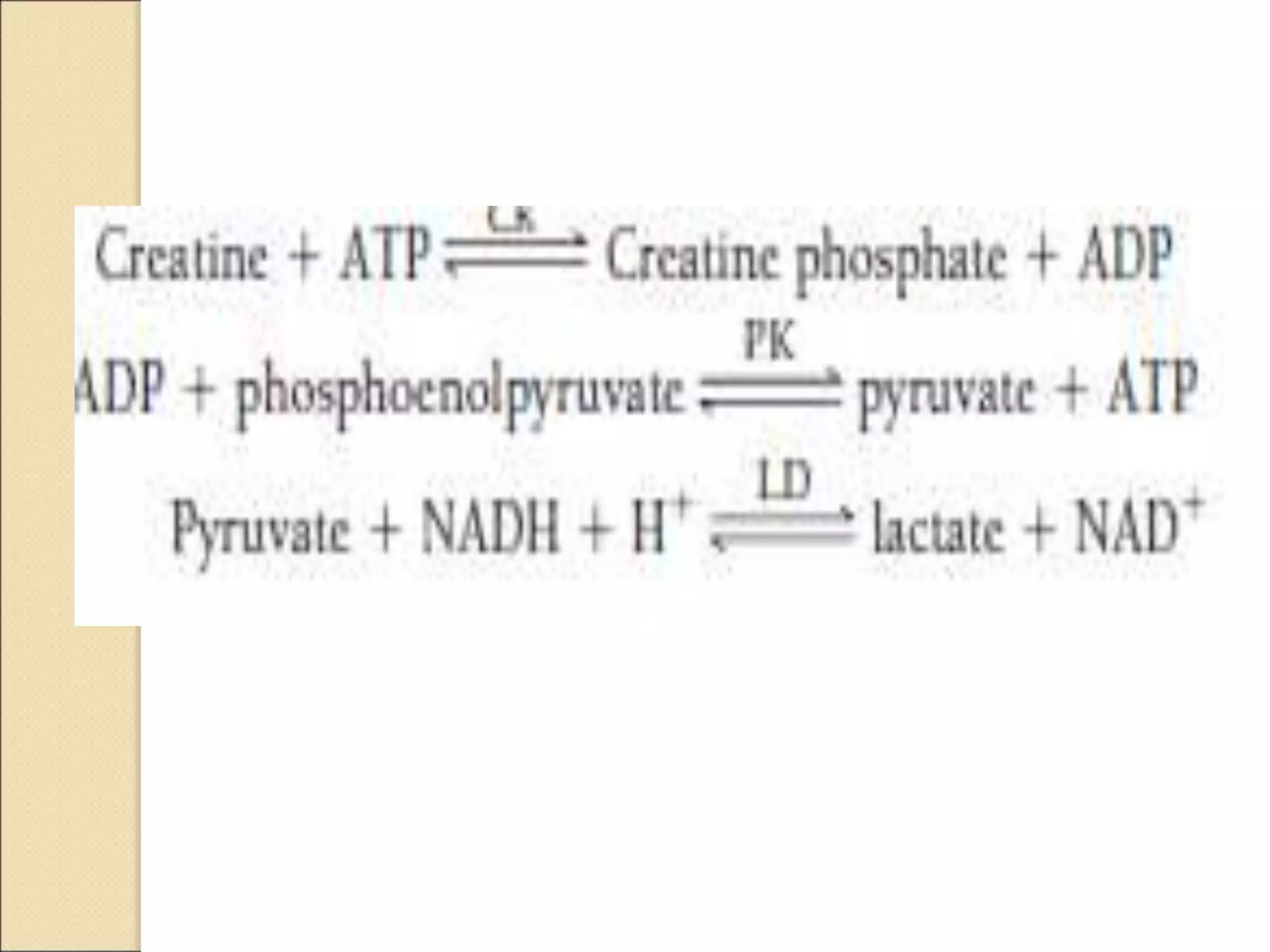
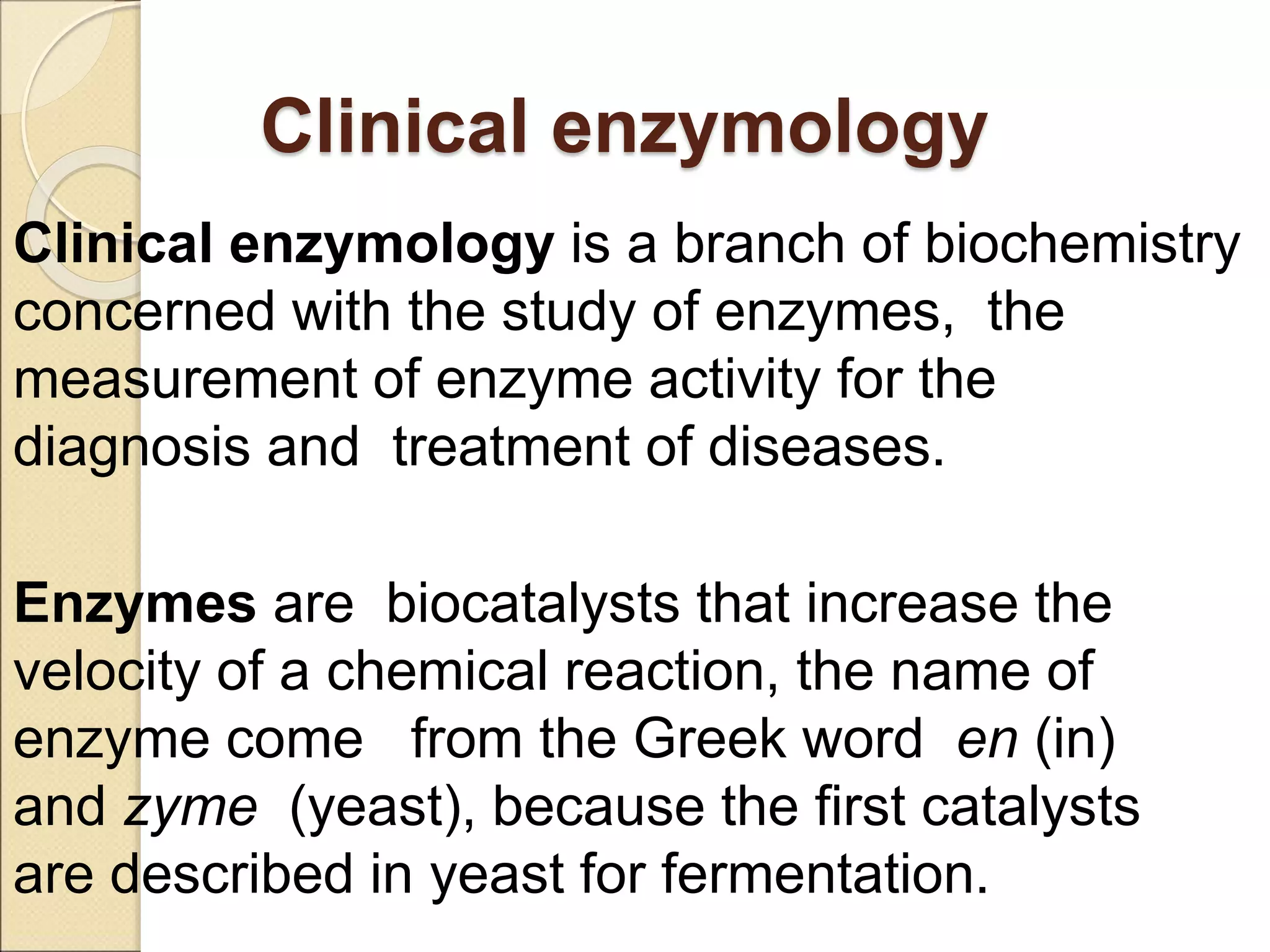
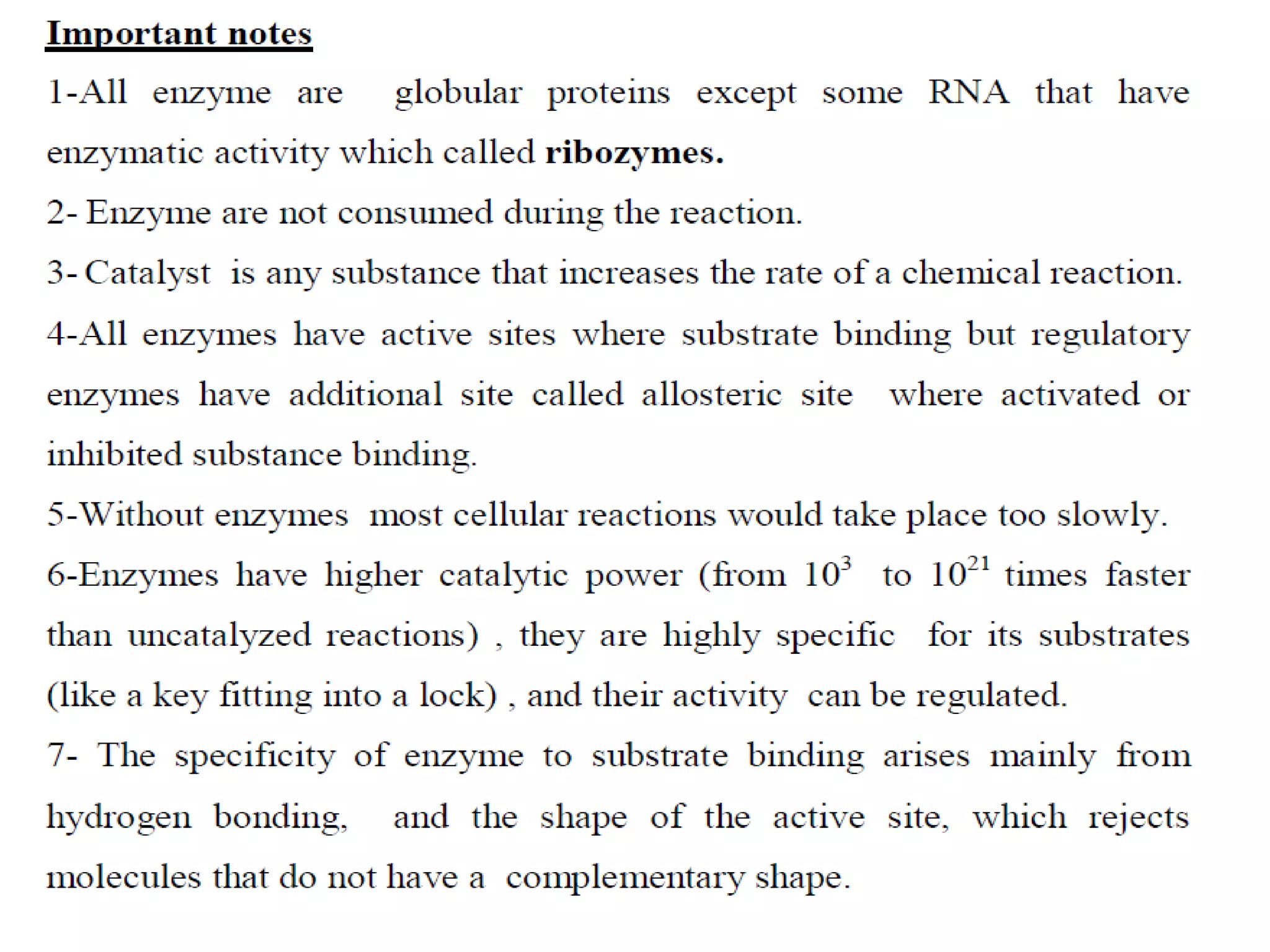
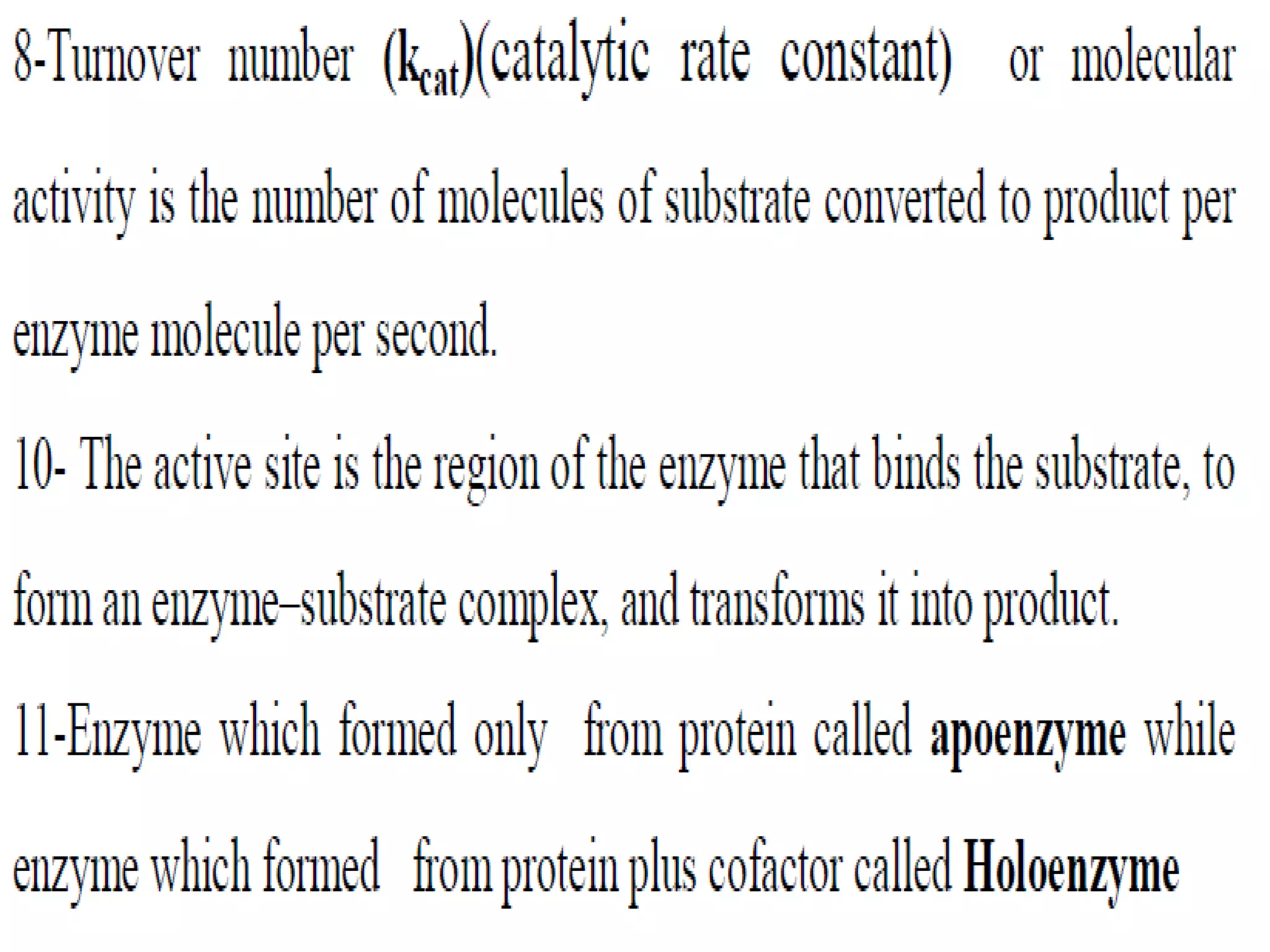
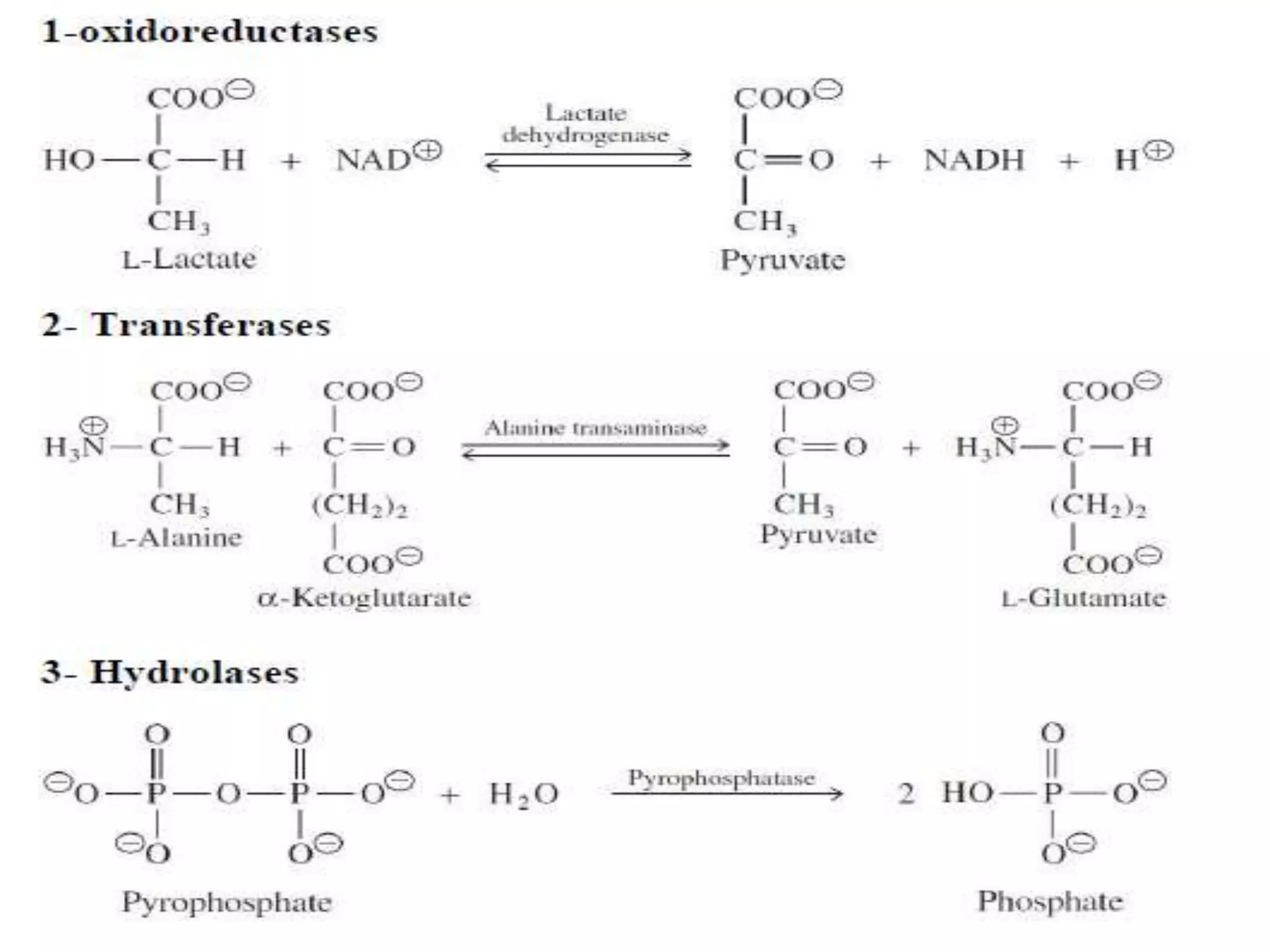
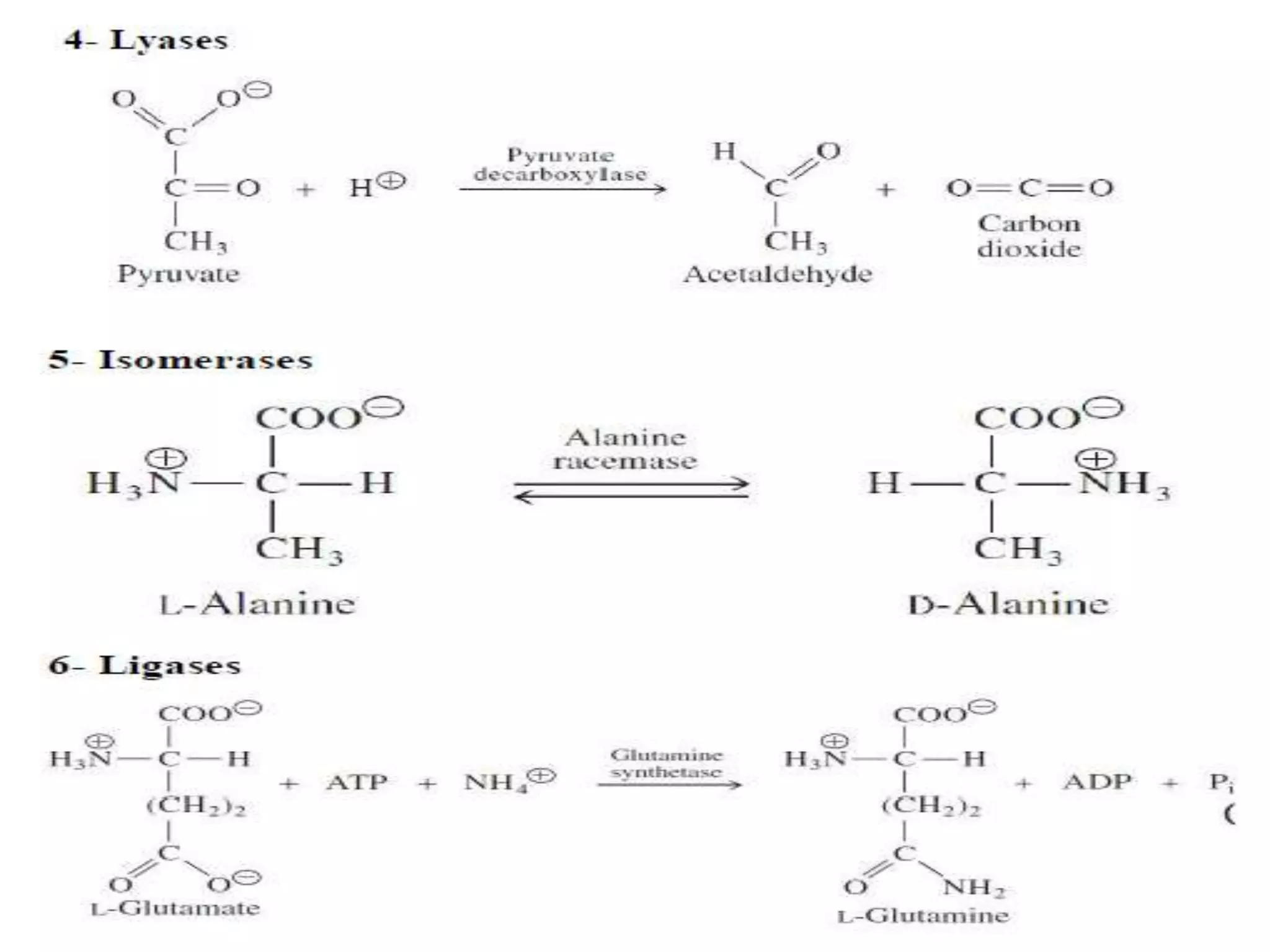
This document discusses clinical enzymology and enzymes of clinical significance. It provides information on several key enzymes including ALT, AST, ALP, GGT, and α-amylase. ALT and AST are liver enzymes that are elevated in liver damage or disease. ALP is also elevated in liver diseases but also bone diseases. GGT is specific to liver and pancreas and useful for monitoring alcohol use. α-Amylase is produced in the pancreas and salivary glands and elevated in pancreatitis. The document discusses the normal ranges, sources of error, and causes of increased levels for several important clinical enzymes.



![Factors affecting the activity of enzymes
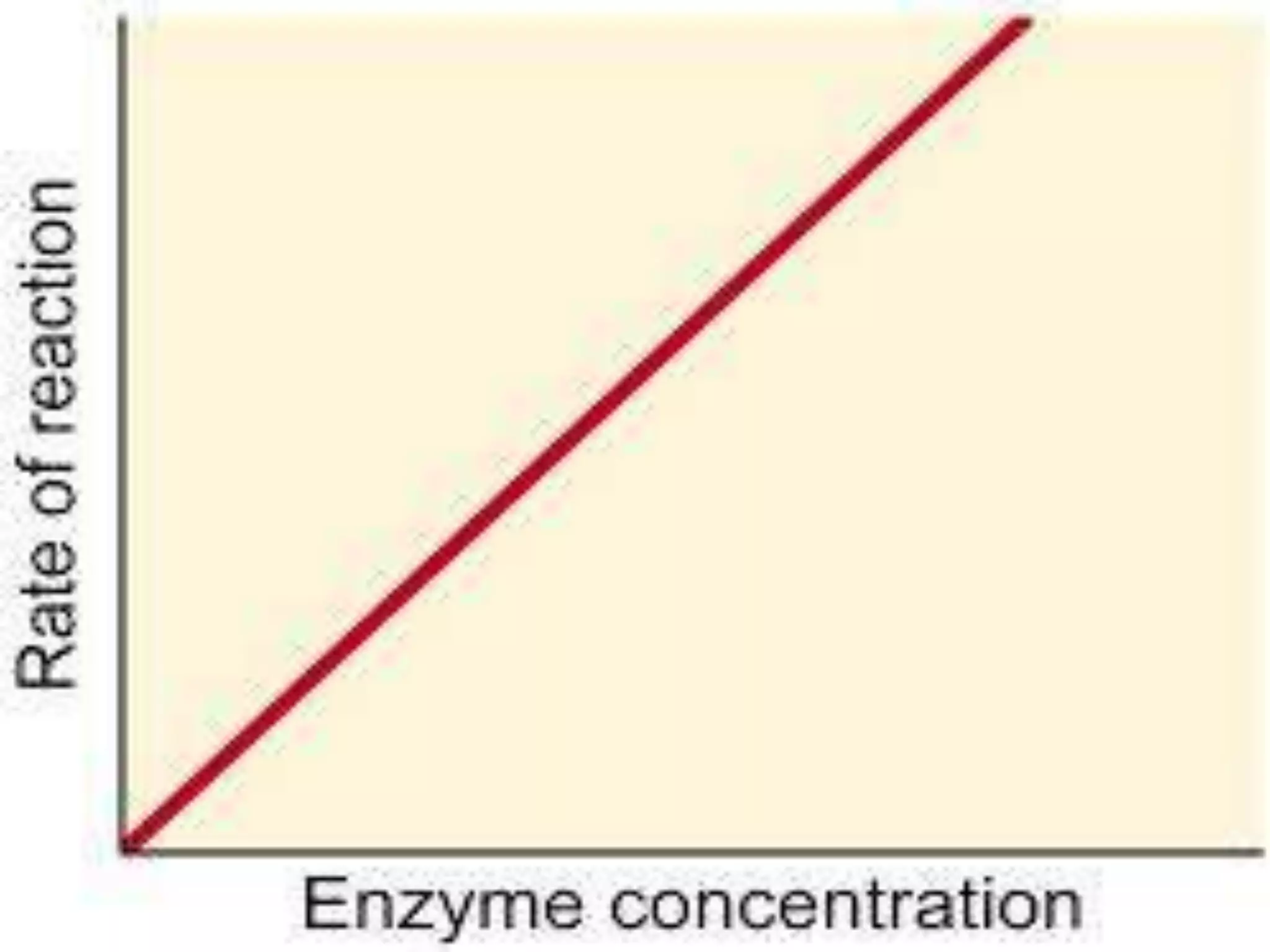
1-Enzyme Concentration [ E ]](https://image.slidesharecdn.com/clinicalenzymology-221124105646-9fc9009f/75/Clinical-enzymology-ppt-9-2048.jpg)
![2-Substrate concentration [S]](https://image.slidesharecdn.com/clinicalenzymology-221124105646-9fc9009f/75/Clinical-enzymology-ppt-11-2048.jpg)