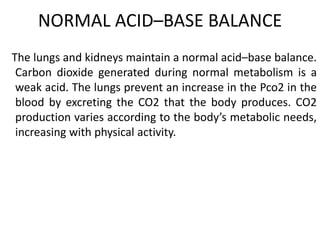
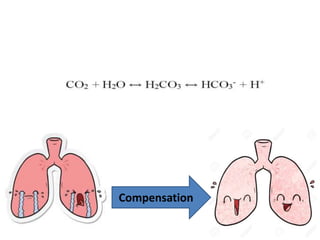
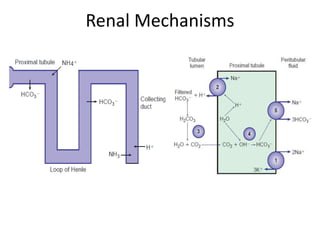
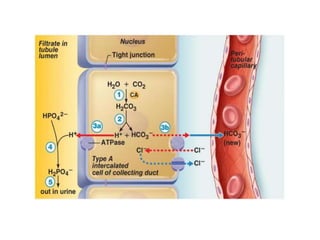
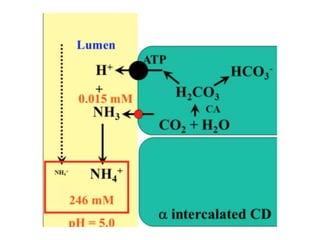
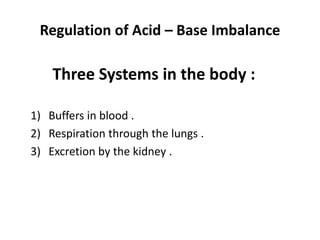
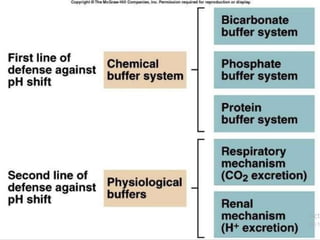
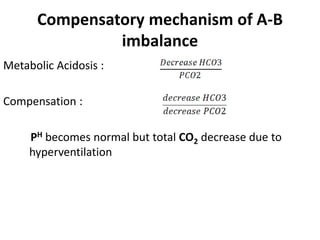
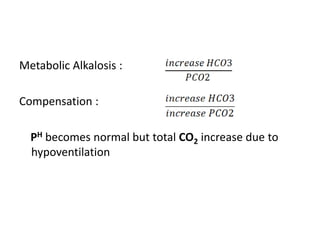
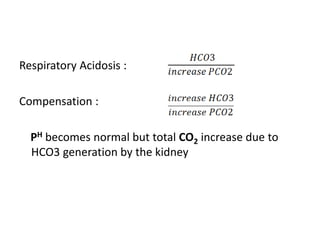
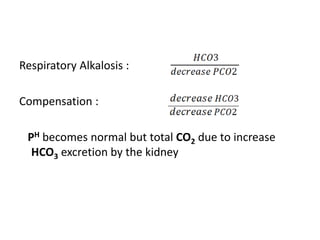
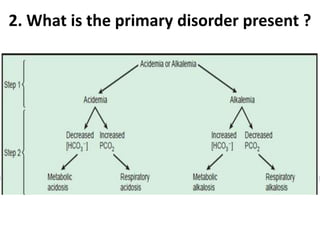
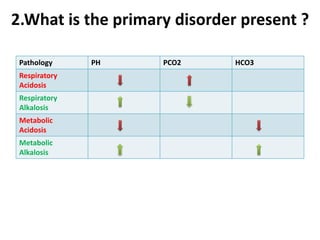
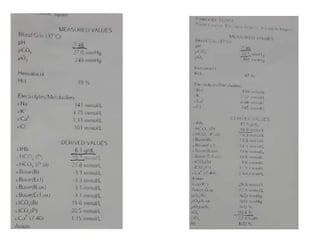
The document presents a seminar on acid-base physiology, emphasizing the importance of maintaining pH levels in the body through the regulation of hydrogen and bicarbonate ions. It discusses the role of the lungs and kidneys in managing acid-base balance, common acid-base disorders, and their causes, particularly in newborns. Additionally, it covers arterial blood gas (ABG) analysis as a critical tool for assessing acid-base status in patients, particularly in intensive care settings.
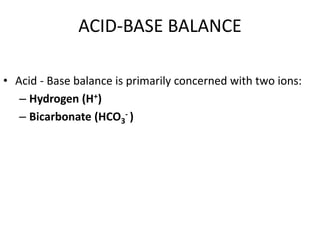
![ Negative log of the hydrogen ion concentration
Thus, pH = - log [H+ ]
pH](https://image.slidesharecdn.com/abg1-200223125126/85/ABG-6-320.jpg)