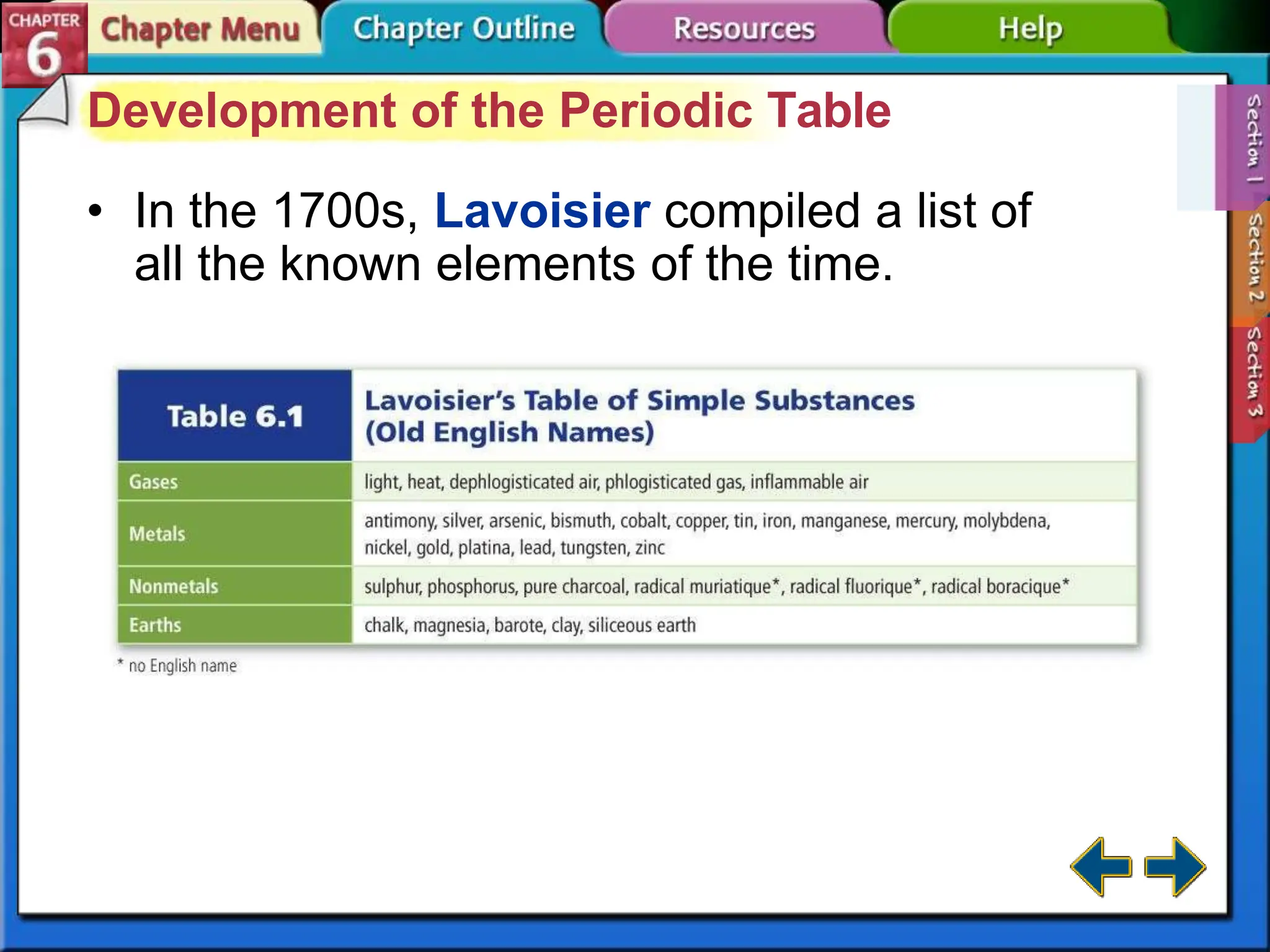
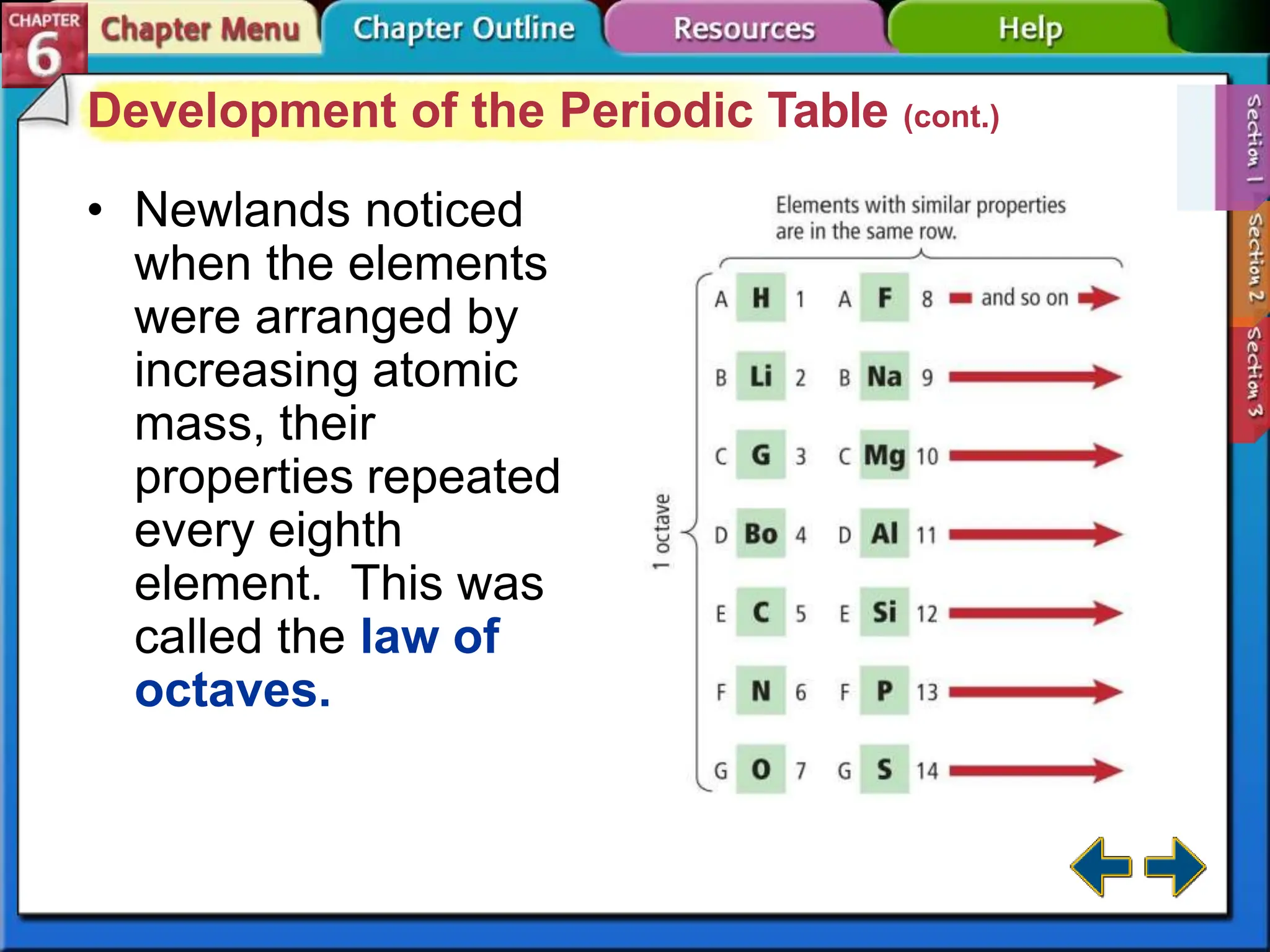
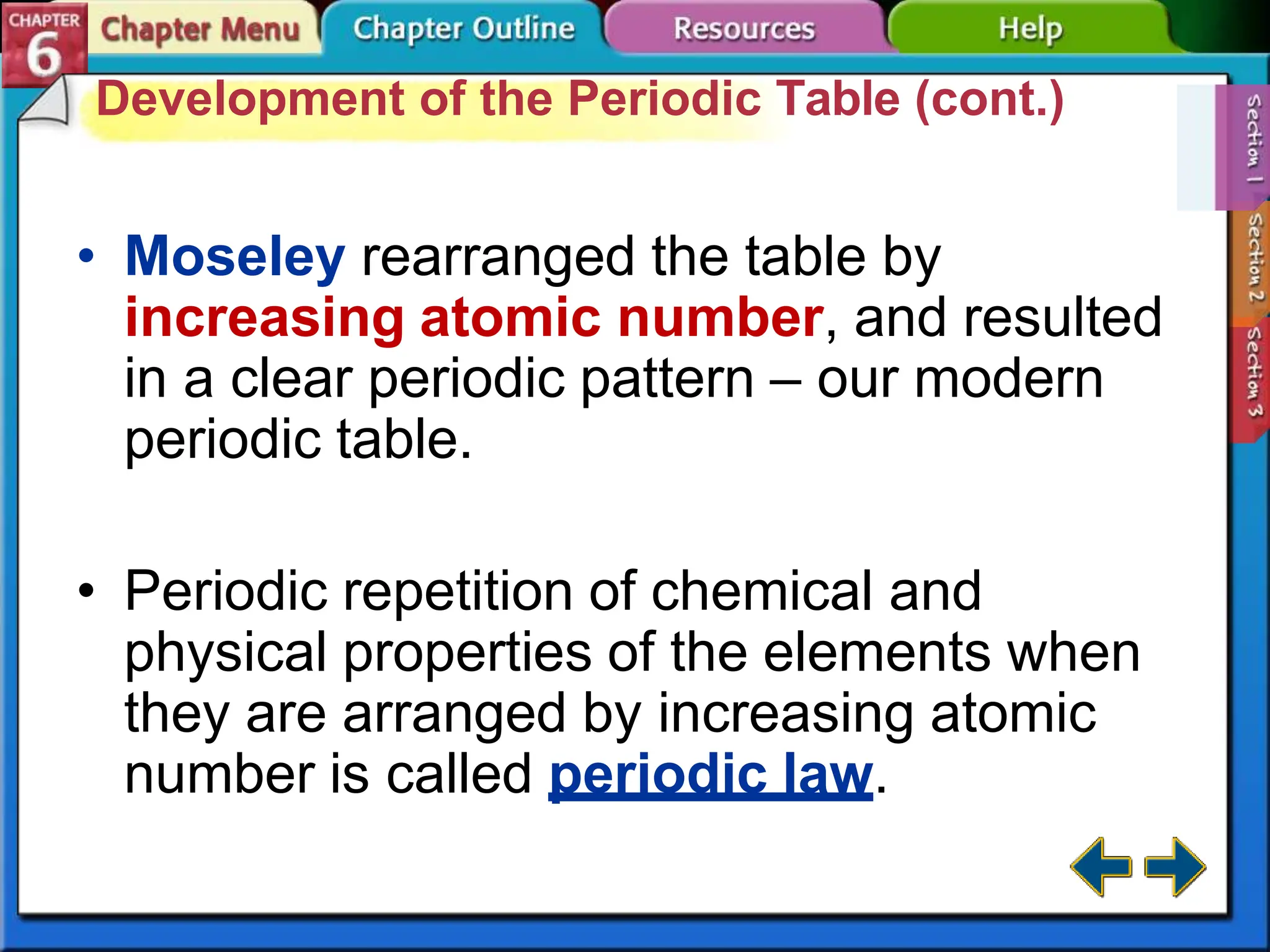
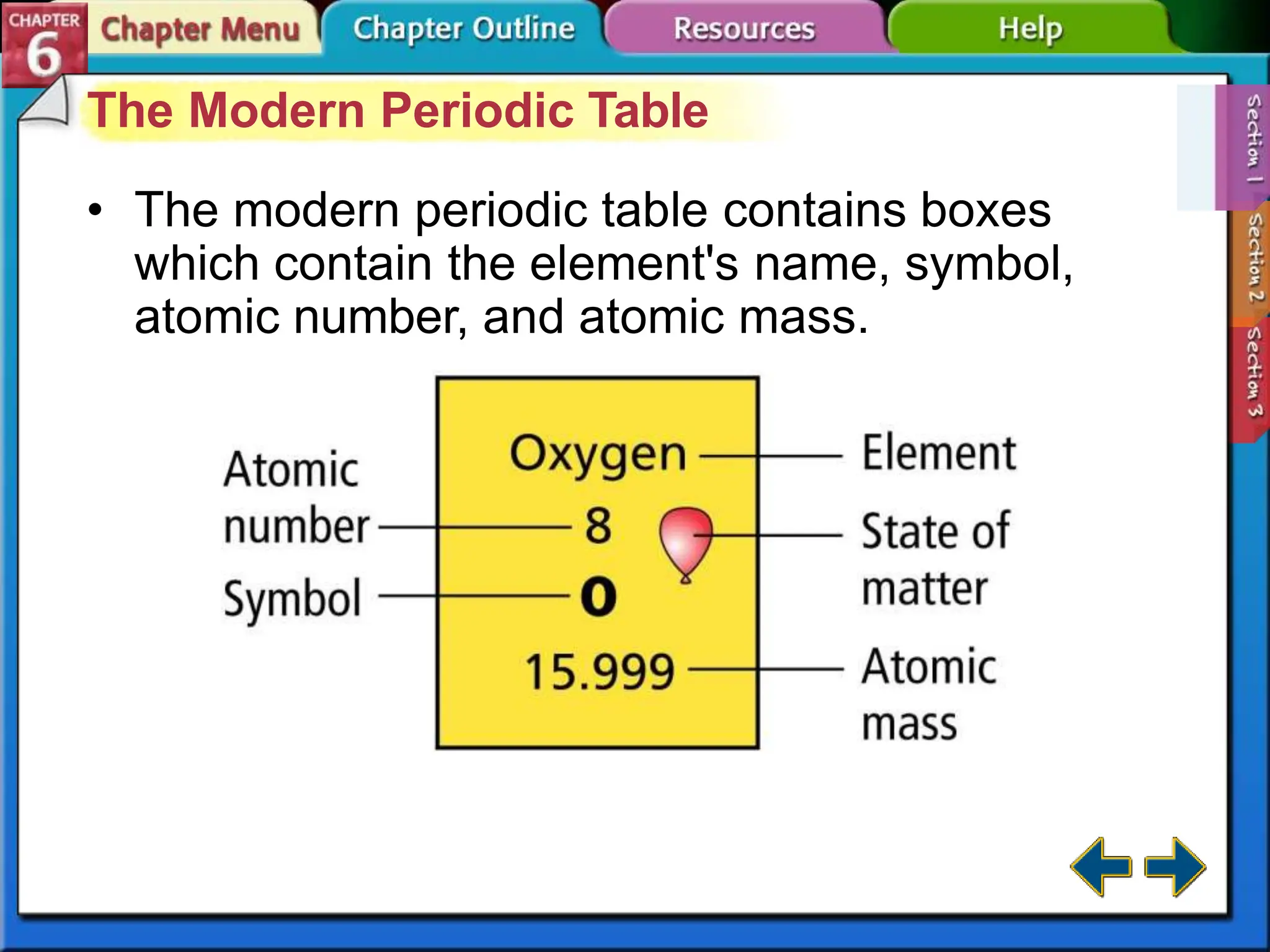
The periodic table evolved over time as scientists discovered more useful ways to organize the elements. John Newlands proposed arranging elements by atomic mass and noticed properties repeated every eighth element, known as the law of octaves. Meyer and Mendeleev connected atomic mass to properties and Mendeleev predicted undiscovered elements. Moseley rearranged the table by atomic number, resulting in the modern periodic table with periodic repetition of properties. The modern table contains element names, symbols, atomic numbers, and masses, arranged in groups and periods.