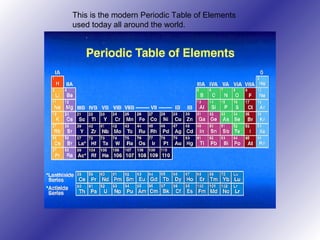
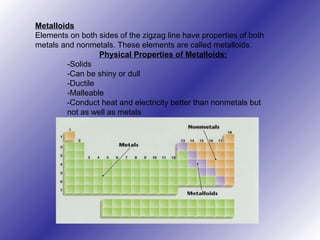
The periodic table of elements has evolved over time through the work of multiple scientists. Dmitri Mendeleev is considered the creator of the modern periodic table in 1869, arranging elements by atomic mass and leaving gaps for undiscovered elements. Later, Henry Moseley determined that atomic number, not mass, was the fundamental property determining an element's position. The modern periodic table arranges elements in periods and groups based on their atomic structure and properties, categorizing them as metals, nonmetals, metalloids and noble gases.