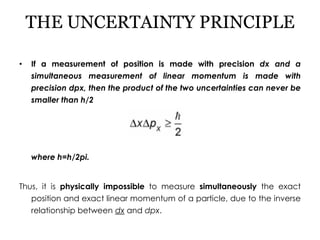
Werner Heisenberg developed the uncertainty principle, which states that the more precisely the position of a particle is determined, the less precisely its momentum can be known, and vice versa. This stems from the quantum nature of matter, where measuring devices disturb the system being measured. A thought experiment is described where observing an electron's position with a photon impacts the electron's momentum in an unpredictable way. The uncertainty principle is expressed as ΔxΔp≥h/2π, meaning the product of the uncertainties in position and momentum must be greater than or equal to Planck's constant divided by 2π.