The document discusses the uncertainty principle in quantum mechanics. It makes three key points:
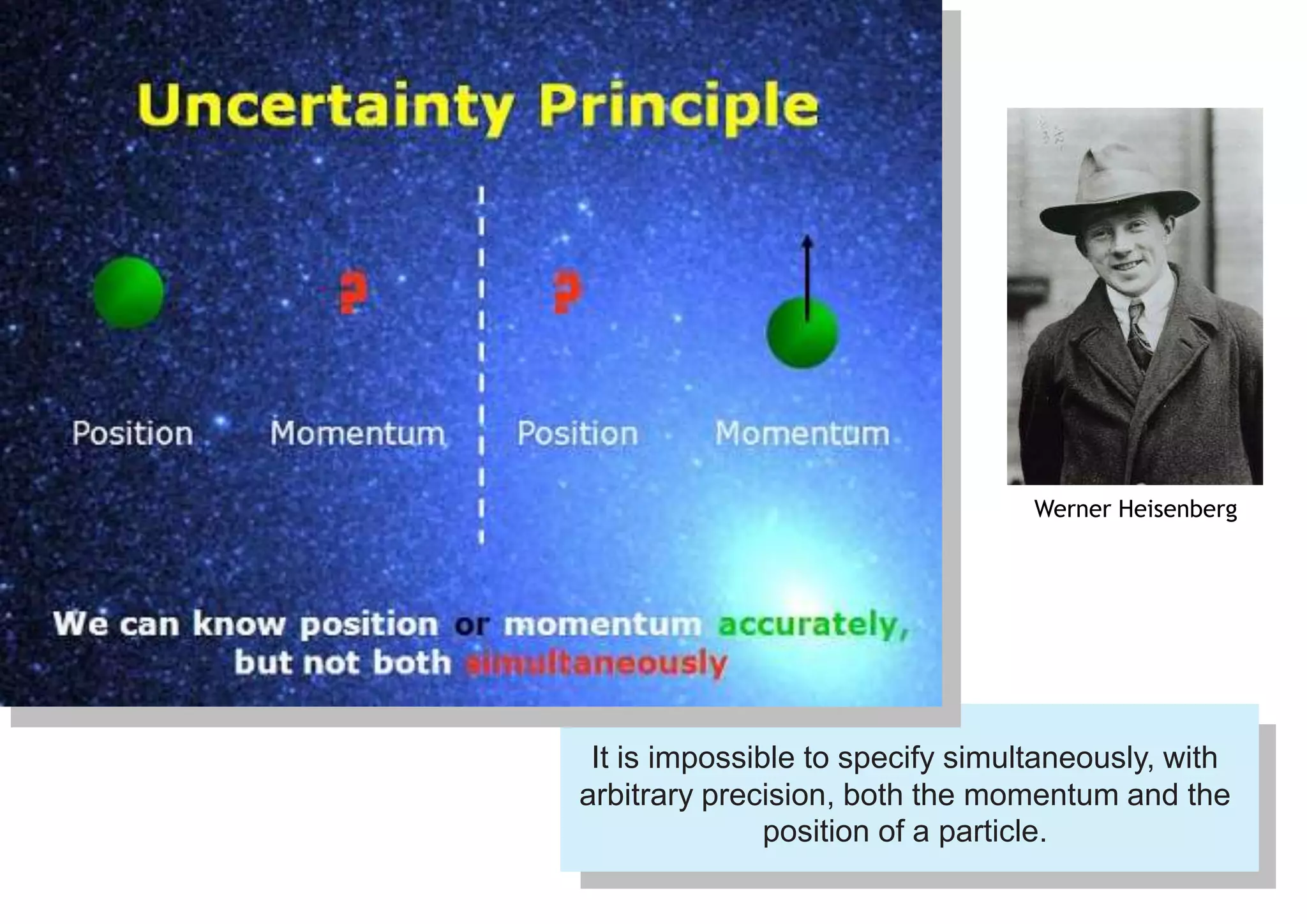
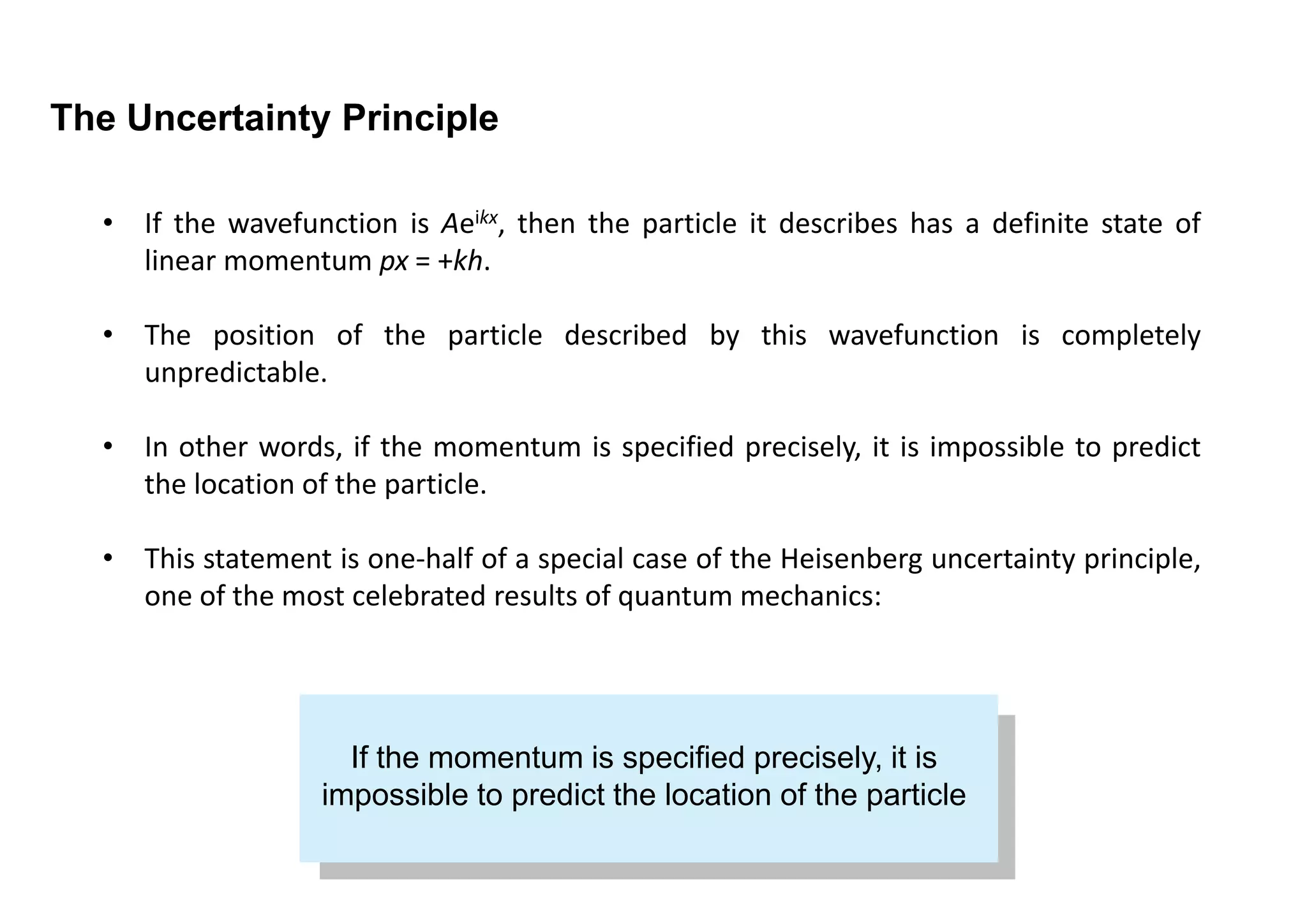
1) It is impossible to simultaneously measure the exact position and momentum of a particle. If one property is known precisely, the other cannot be predicted at all.
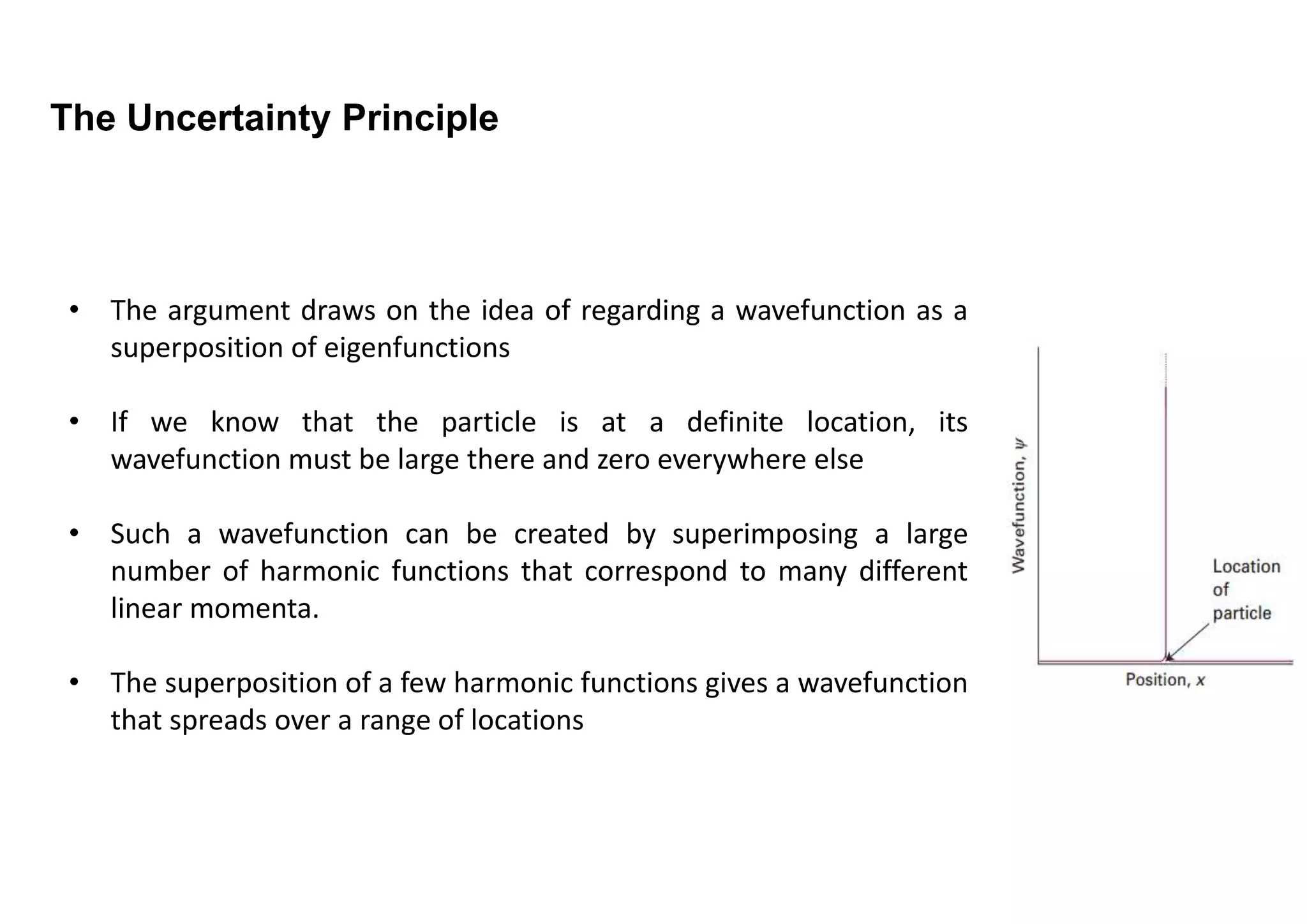
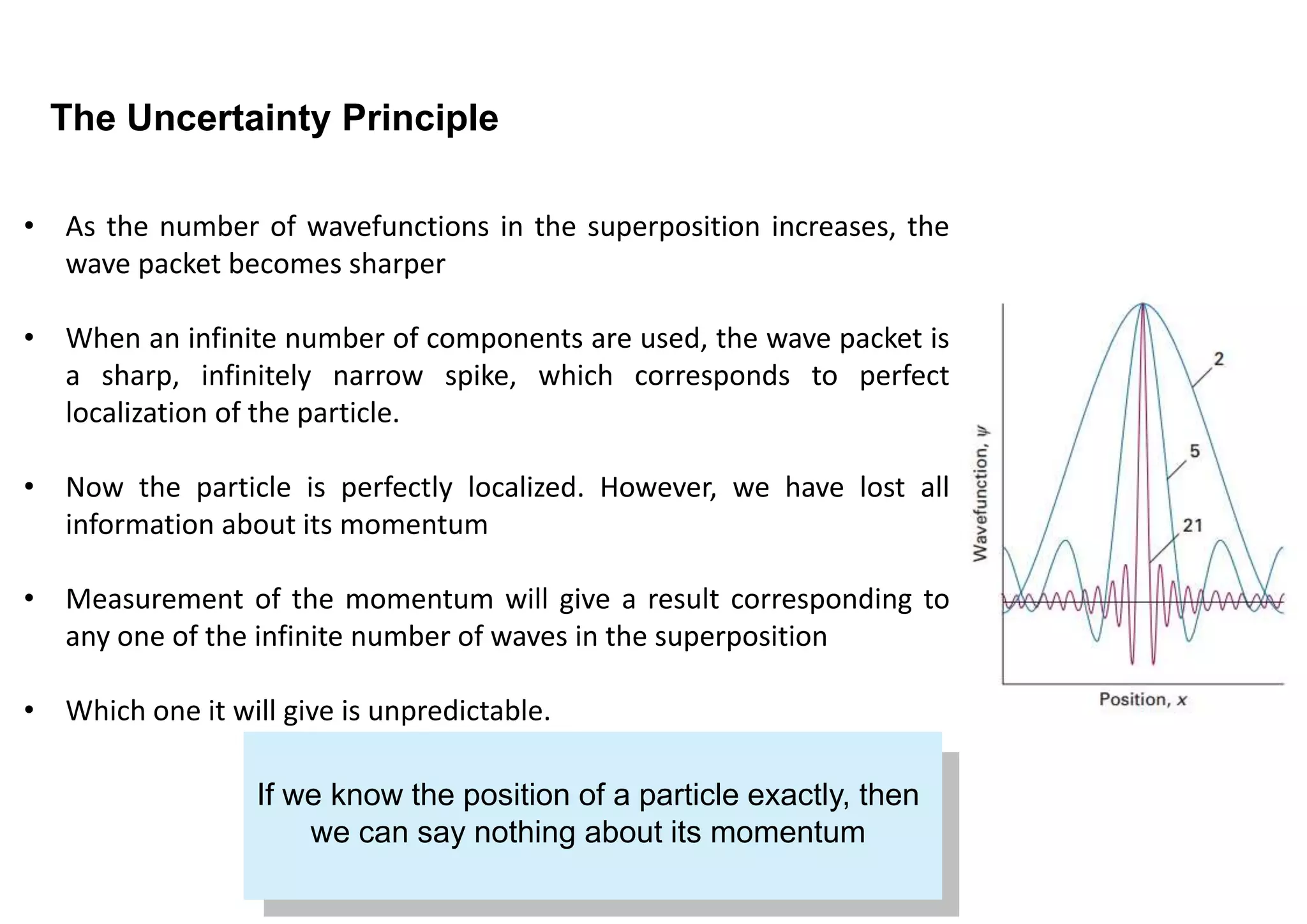
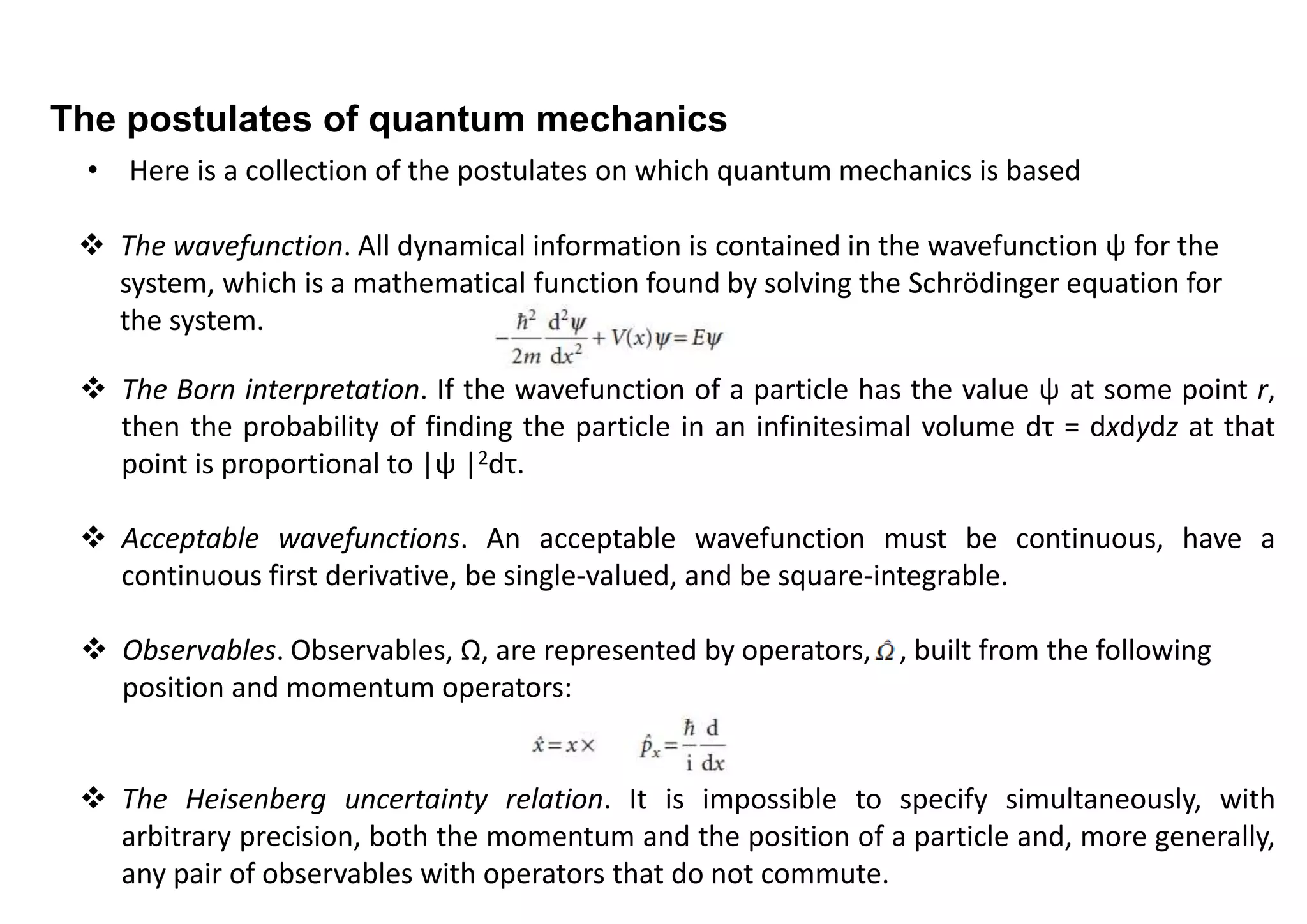
2) The uncertainty principle arises due to the wave-like nature of particles. A particle's position is described by a wavefunction, and knowing its exact position would require an infinite number of waves with different momenta.
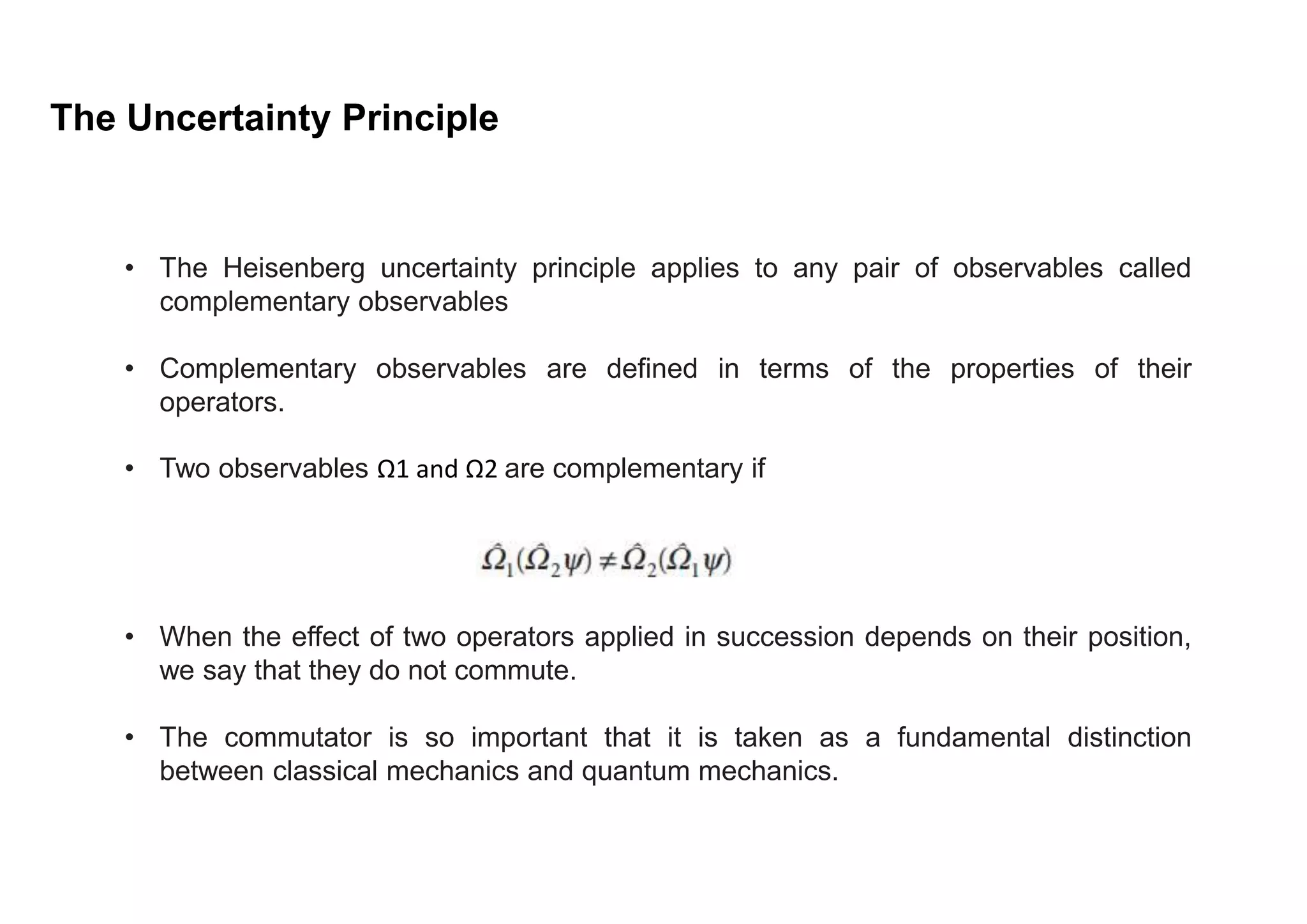
3) The uncertainty principle applies broadly to any pairs of "complementary observables" with non-commuting operators, not just position and momentum. It represents a fundamental difference between classical and quantum mechanics.