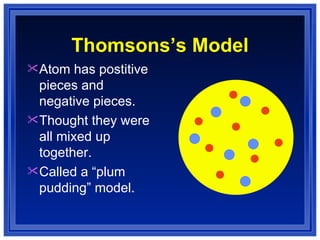
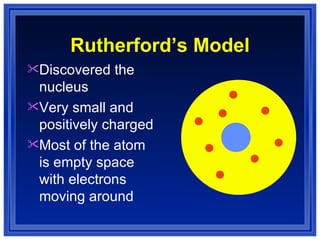
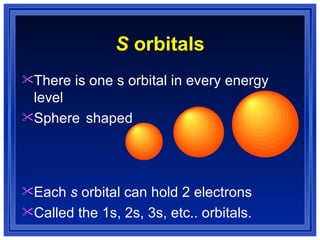
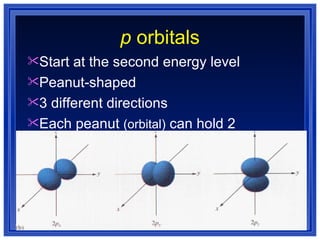
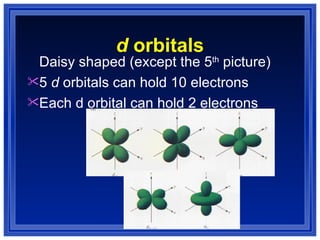
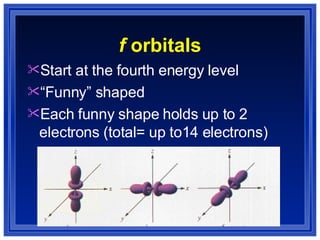
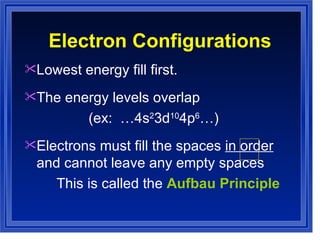
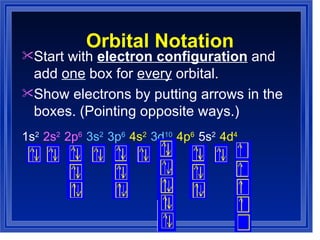
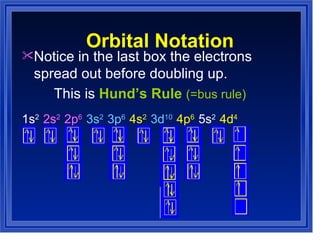
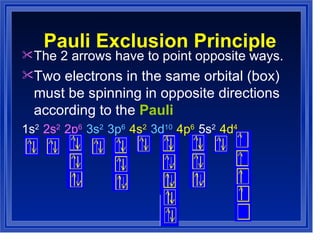
This document summarizes key models of the atom and concepts about electrons in atoms. It discusses Thomson's "plum pudding" model, Rutherford's discovery of the nucleus, Bohr's model of electrons in energy levels, and the modern quantum mechanical model. It also describes electron configurations, orbital notation, Hund's rule, the Aufbau principle, and the Pauli exclusion principle.