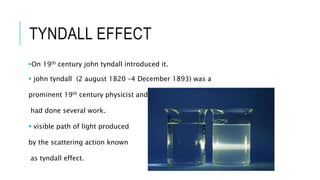
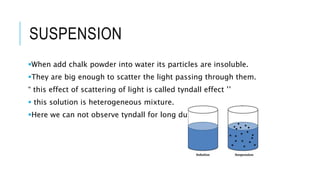
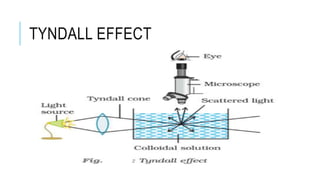
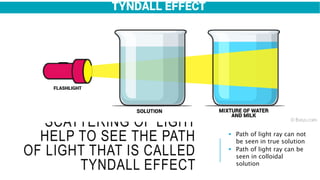
The document discusses the Tyndall effect, which is the scattering of light by colloidal particles or molecules in a suspension, causing the light path to be visible. It provides:
1) A brief history of the Tyndall effect and its discovery by John Tyndall in the 19th century.
2) An explanation of the differences between solutions, suspensions, and colloids, and how the Tyndall effect can be observed in colloids due to their particle size of 1-100nm.
3) Applications of the Tyndall effect including why the sky appears blue and red at sunrise/sunset.