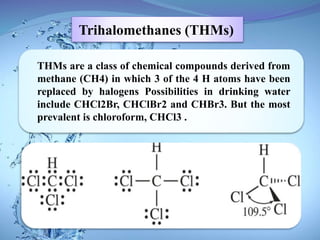
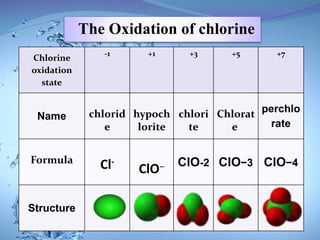
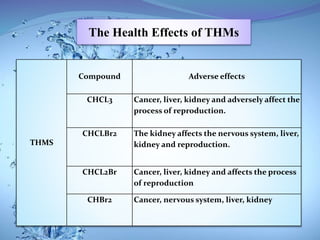
The document discusses the process of chlorination in water treatment, detailing its advantages and disadvantages, particularly the formation of trihalomethanes (THMs) which can be harmful to human health. It also covers perchlorate contamination and its health effects, including links to cancer and reproductive issues. Recommendations for reducing THM formation and managing organic material in water are provided, including the use of alternative disinfection methods.