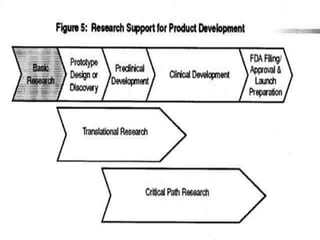
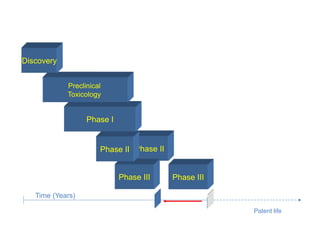
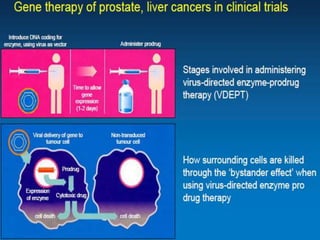
Translational research aims to bridge the gap between basic science discoveries and clinical applications to improve human health. It involves translating findings from laboratory and preclinical studies into potential new diagnostics and treatments for patients. There are multiple phases of translational research, including T1 which moves basic discoveries into clinical applications, and T2 which provides evidence of a discovery's value in a clinical setting. Effective translation is needed to realize the benefits of increased biomedical research funding and requires tools like biomarkers to facilitate predicting efficacy and safety across species.