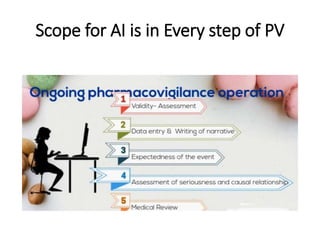
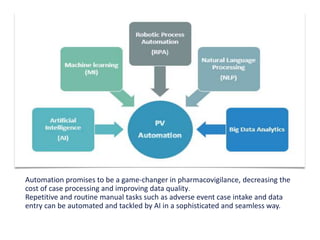
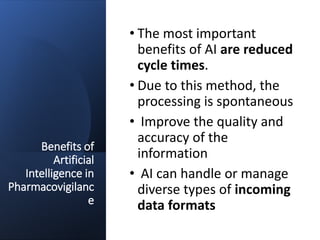
Artificial intelligence can help improve pharmacovigilance in three key ways:
1) AI can automate repetitive manual tasks like data entry of adverse event reports to improve efficiency.
2) Machine learning algorithms can be trained on large databases of adverse drug reaction reports to help identify new safety signals.
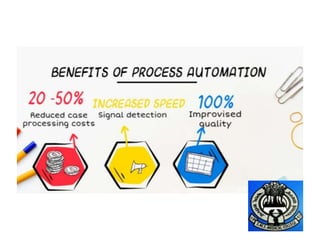
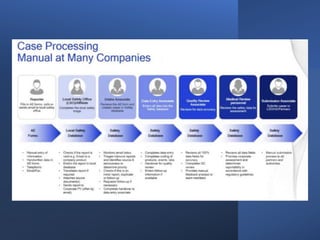
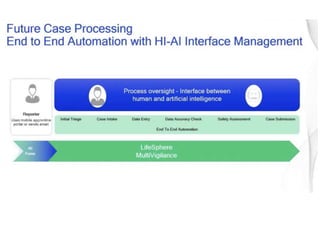
3) AI tools show promise in extracting clinically relevant information from individual case safety reports without human review, reducing the time spent on case processing. However, challenges remain around data quality, costs, and ensuring AI augmentation rather than replacement of human experts.



















![Research Projects
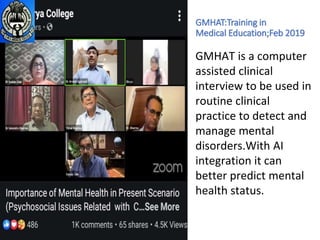
[15]& GMHAT
1. Mental health assessment by medical
students of Batch 2016
using GMHAT.
2. Mental health assessment by medical
undergraduate of Batch 2017
students using GMHAT.
3.Assessment of Mental health of
participants by MBBS Batch 2018 by
using
GMHAT.
4. To assess and compare the effect of
traditional teaching with Integrated
teaching in MBBS students with the
help of GMHAT this also
included in teaching timetable.
5. Use GMHAT By Postgraduate students
in there training & thesis work on Cancer,
HIV, TB, Diabetes and Osteoarthritis
patients.](https://image.slidesharecdn.com/aiinpv-ppt-final-x1-220520010845-a30c4d04/85/Artificial-intelligence-in-Pharmacovigilance-34-320.jpg)