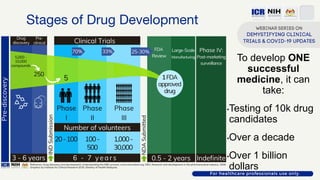
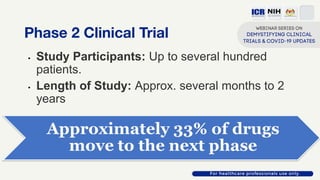
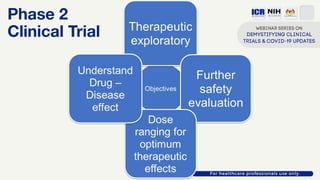
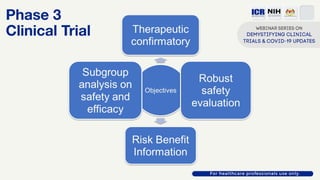
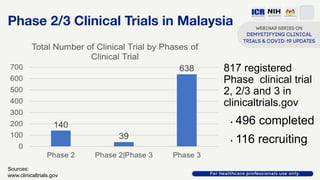
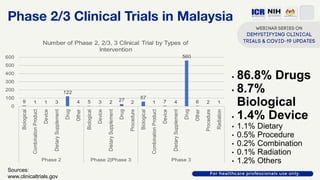
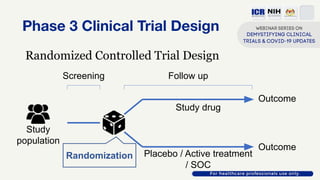
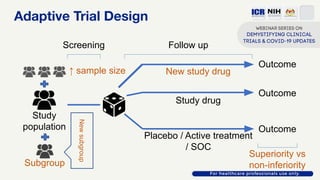
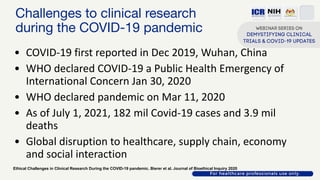
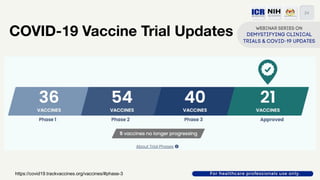
The document discusses Phase 2 and Phase 3 clinical trials, outlining their respective study designs, participant numbers, and purposes. It highlights challenges faced during the COVID-19 pandemic, including resource allocation and ethical considerations, while providing statistics on trials currently registered in Malaysia. Additionally, it emphasizes the importance of designing research that is scientifically valid and ethically sound, especially under public pressure.