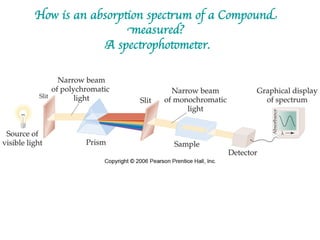
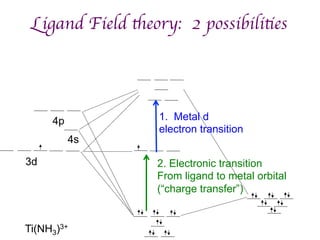
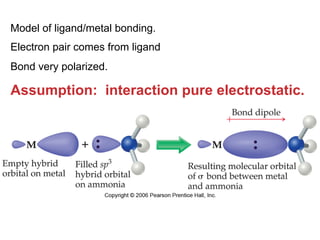
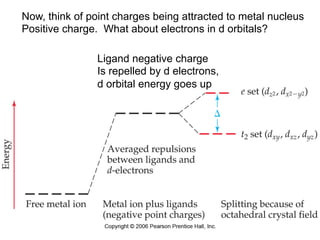
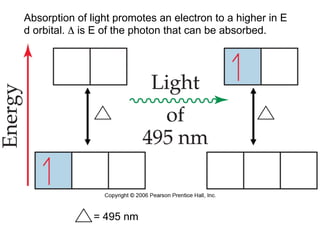
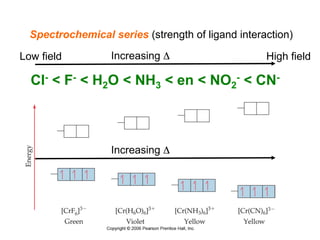
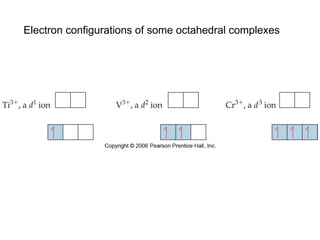
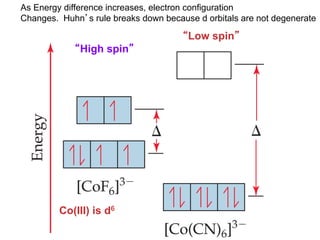
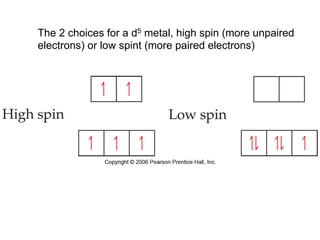
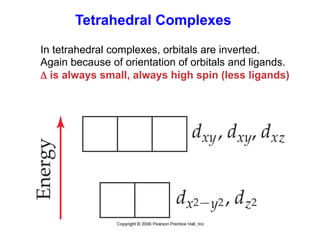
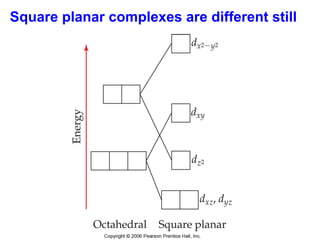
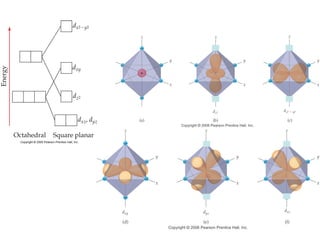
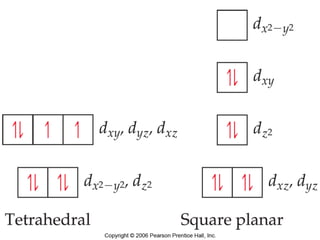
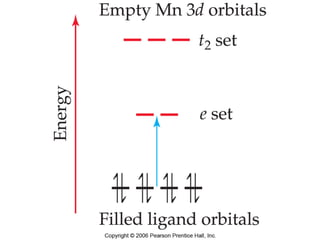
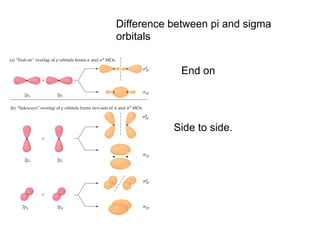
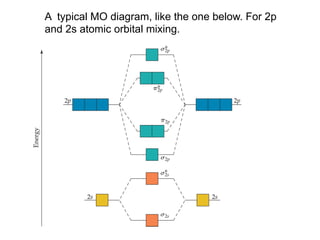
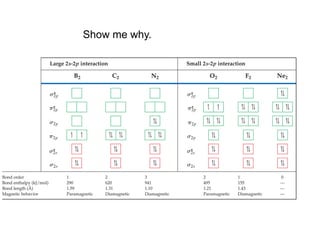
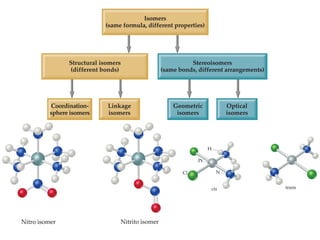
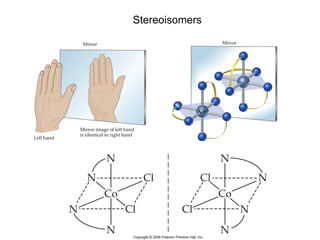
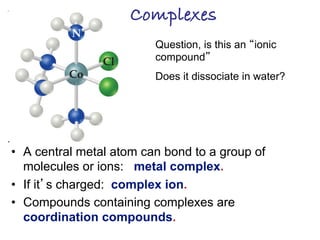
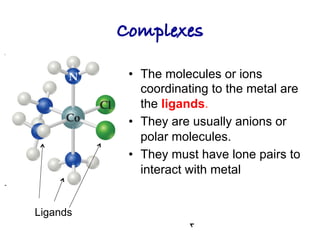
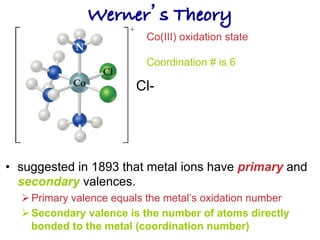
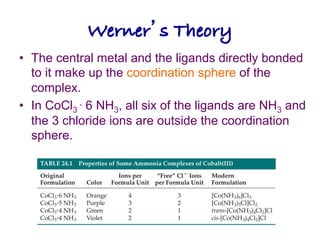
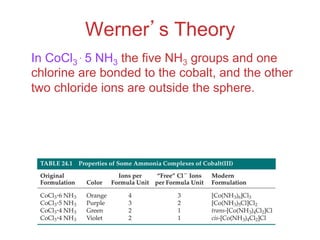
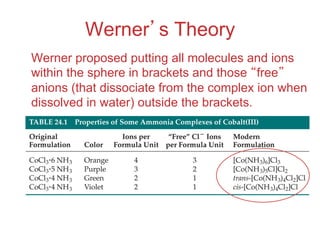
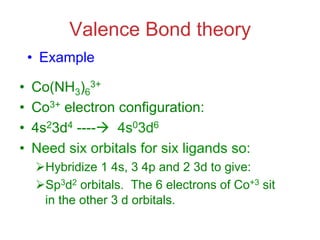
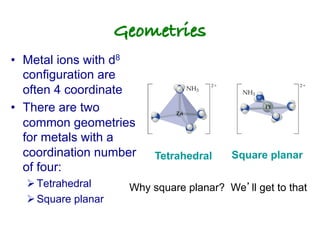
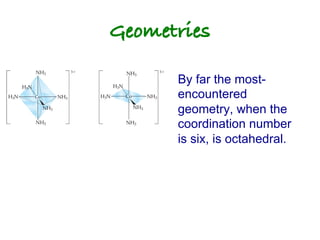
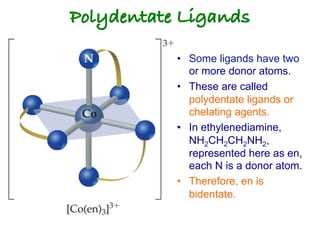
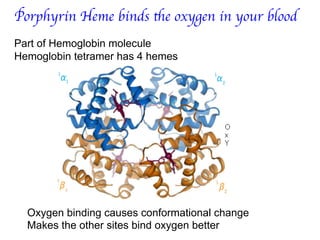
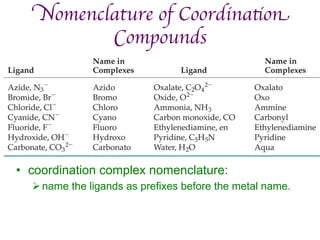
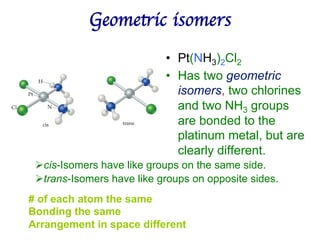
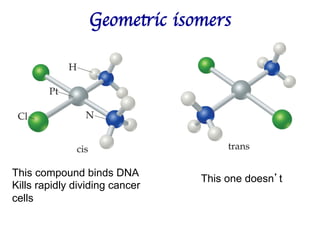
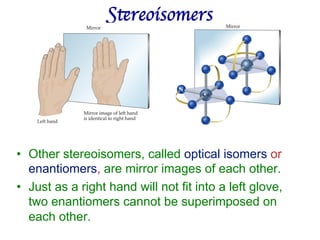
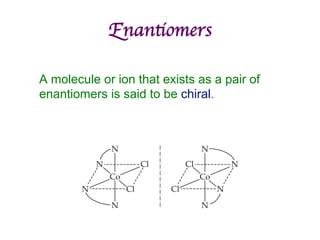
1. Coordination compounds form when transition metals bond to other atoms or molecules.
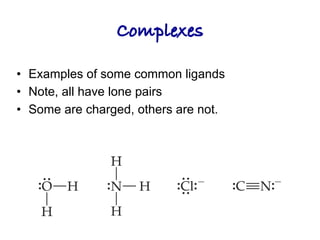
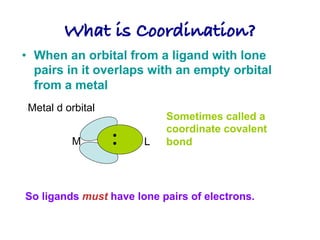
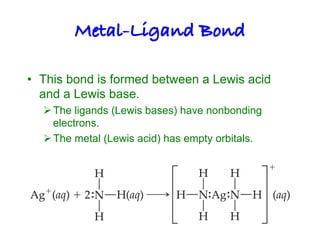
2. Ligands contain lone pairs that can overlap with empty orbitals on the metal to form coordinate covalent bonds.
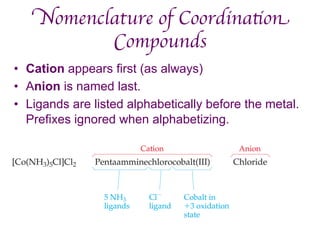
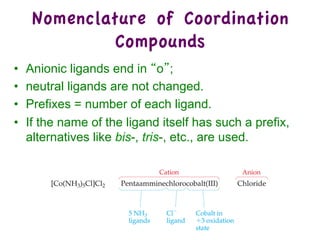
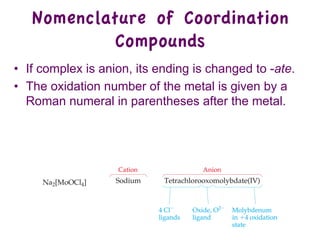
3. Werner's theory proposed that ligands within the coordination sphere be written in brackets, while ligands outside the sphere be written without brackets to predict the number of ions formed.


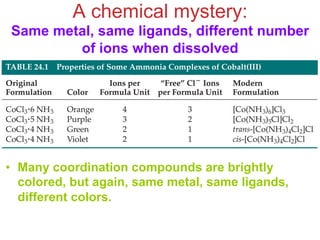
![Werner’s Theory!
• This approach correctly
predicts there would be two
forms of CoCl3 · 4 NH3.
ØThe formula would be written
[Co(NH3)4Cl2]Cl.
ØOne of the two forms has the two
chlorines next to each other.
ØThe other has the chlorines
opposite each other.](https://image.slidesharecdn.com/coordination-221009075611-bdd9468c/85/coordination-pdf-11-320.jpg)



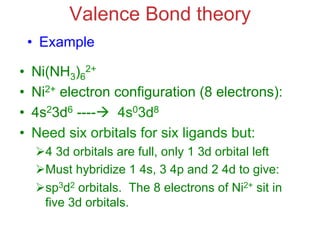

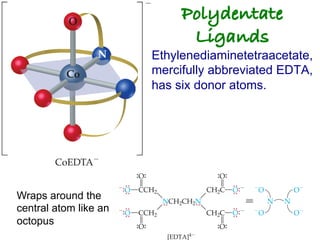
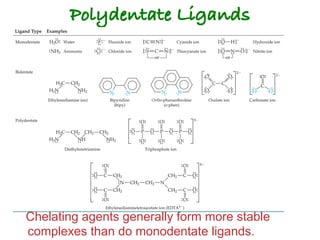
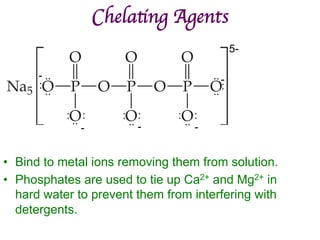

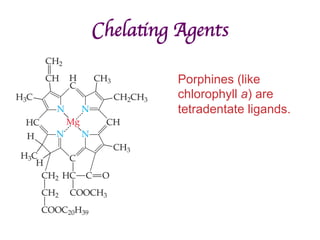
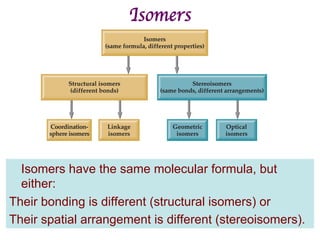
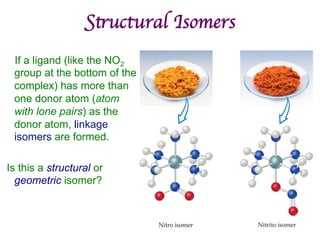
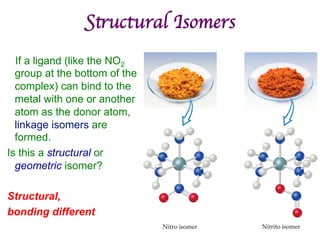
![Structural Isomers
• Some isomers differ in what ligands are
bonded to the metal (coordination
sphere) and which are not.
• these are coordination-sphere isomers.
• Example:
• Three isomers of CrCl3(H2O)6 are
ØThe violet [Cr(H2O)6]Cl3,
ØThe green [Cr(H2O)5Cl]Cl2 ∙ H2O, and
ØThe (also) green [Cr(H2O)4Cl2]Cl ∙ 2 H2O.](https://image.slidesharecdn.com/coordination-221009075611-bdd9468c/85/coordination-pdf-41-320.jpg)




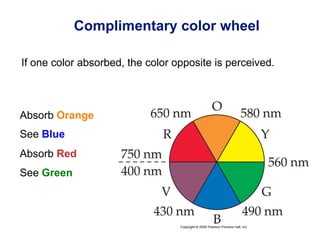
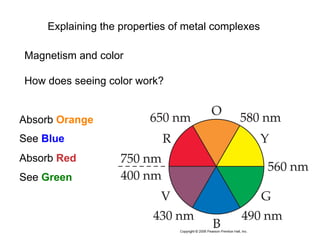
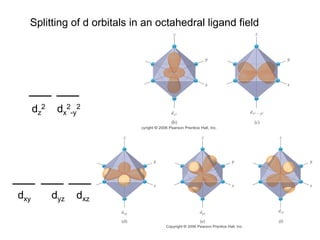
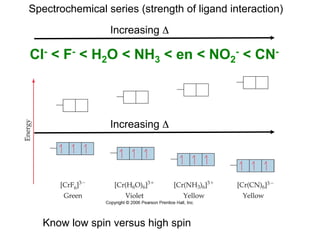
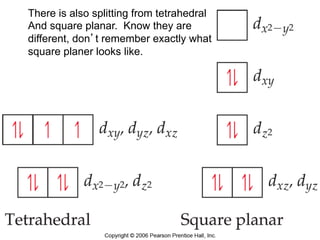
![[Ti(H2O)6]3+
Absorbs in green yellow.
Looks purple.](https://image.slidesharecdn.com/coordination-221009075611-bdd9468c/85/coordination-pdf-53-320.jpg)