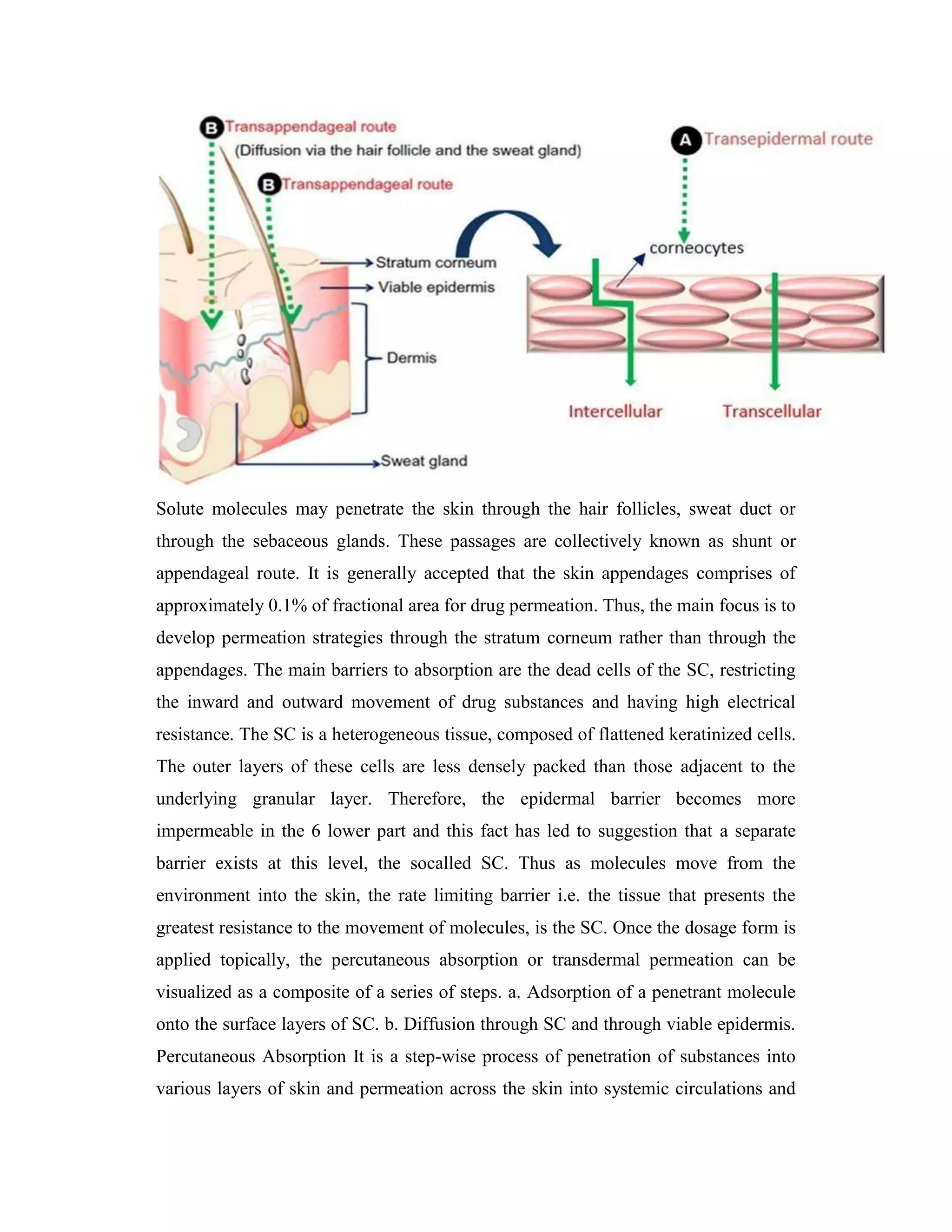
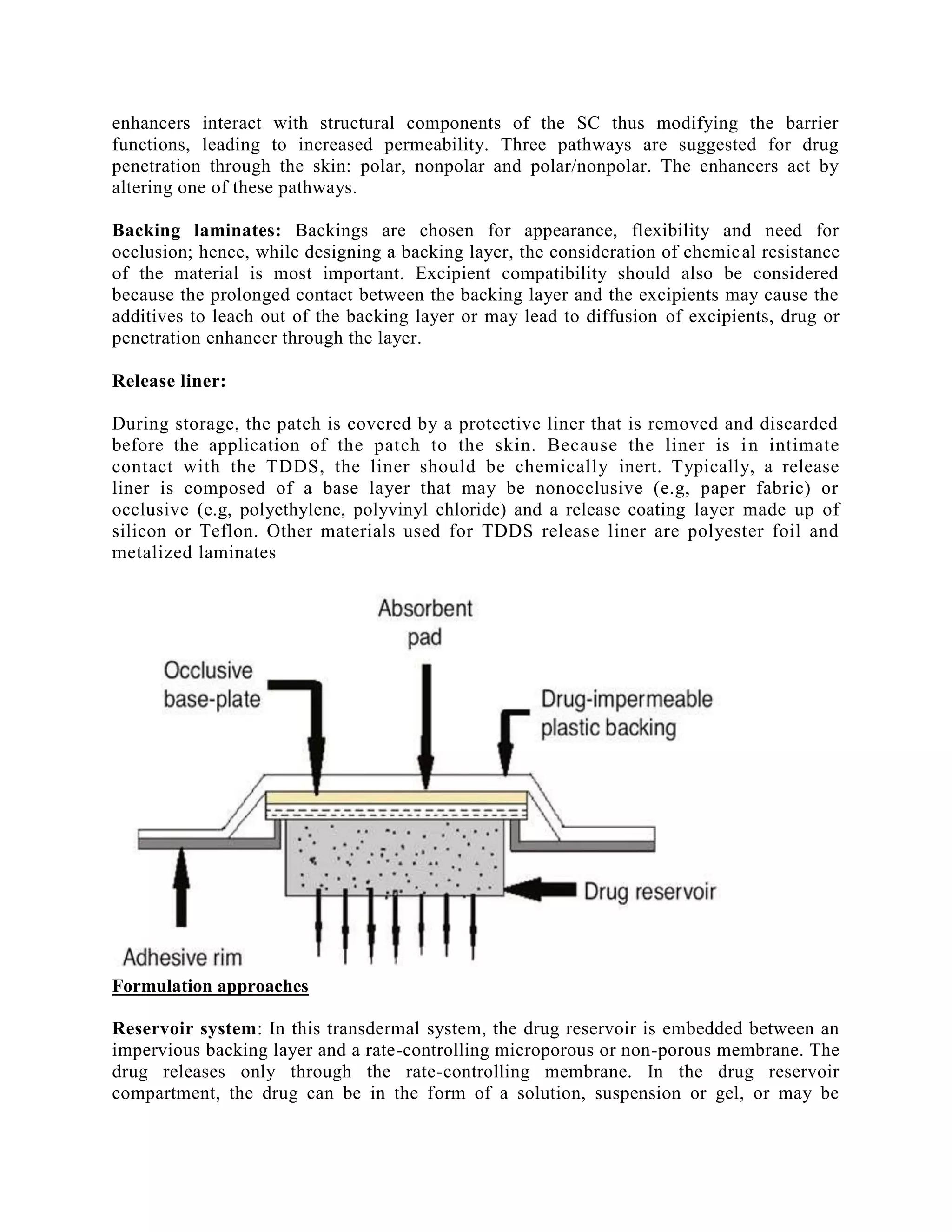
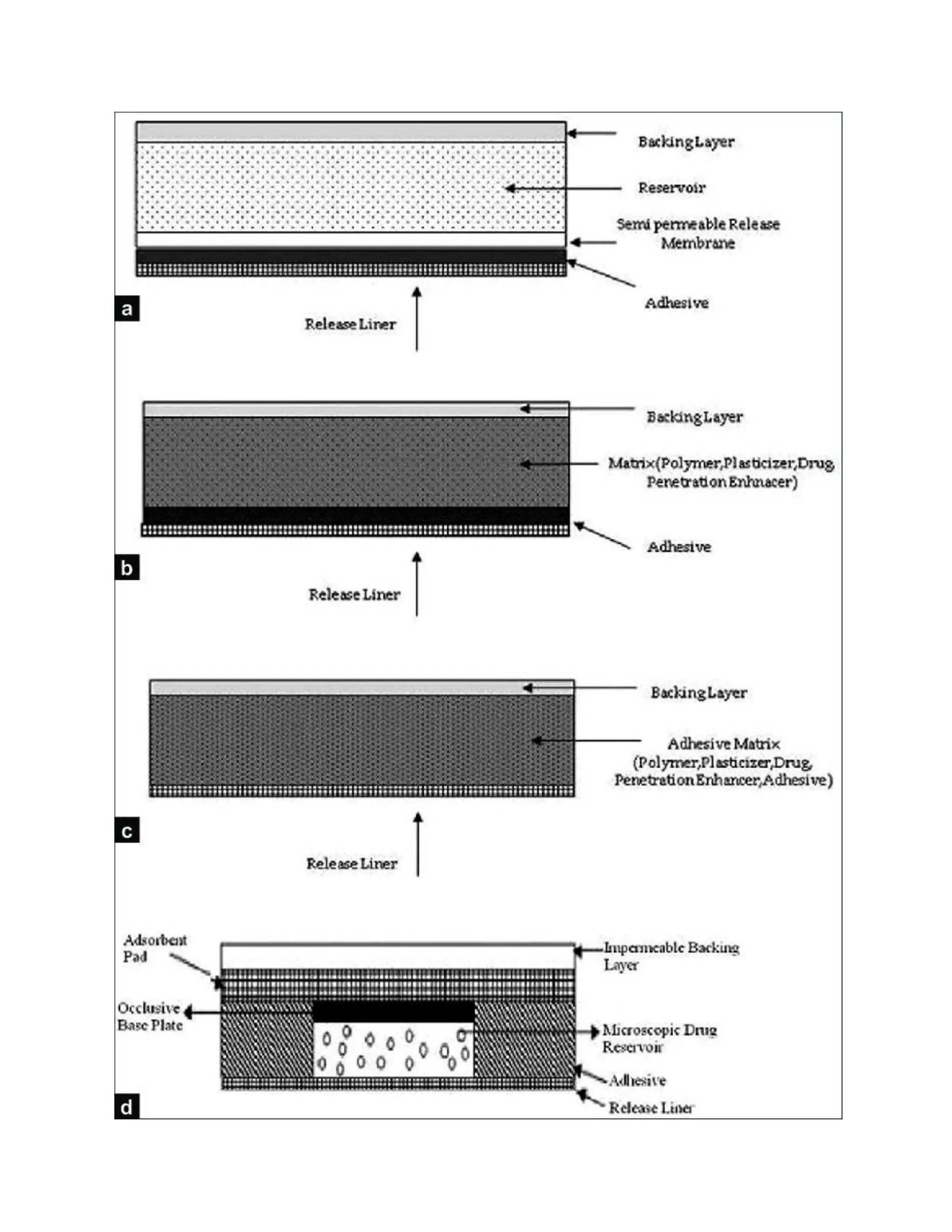
Transdermal drug delivery systems (TDDS), also known as patches, are dosage forms designed to deliver medication through the skin and into the bloodstream. TDDS provides controlled, constant administration of drugs and avoids issues like first-pass metabolism associated with other delivery methods. Key barriers to drug absorption through the skin are the stratum corneum layer and its tight packing of dead skin cells. The rate of permeation is affected by physicochemical properties of the drug like its partition coefficient, molecular size, and solubility.