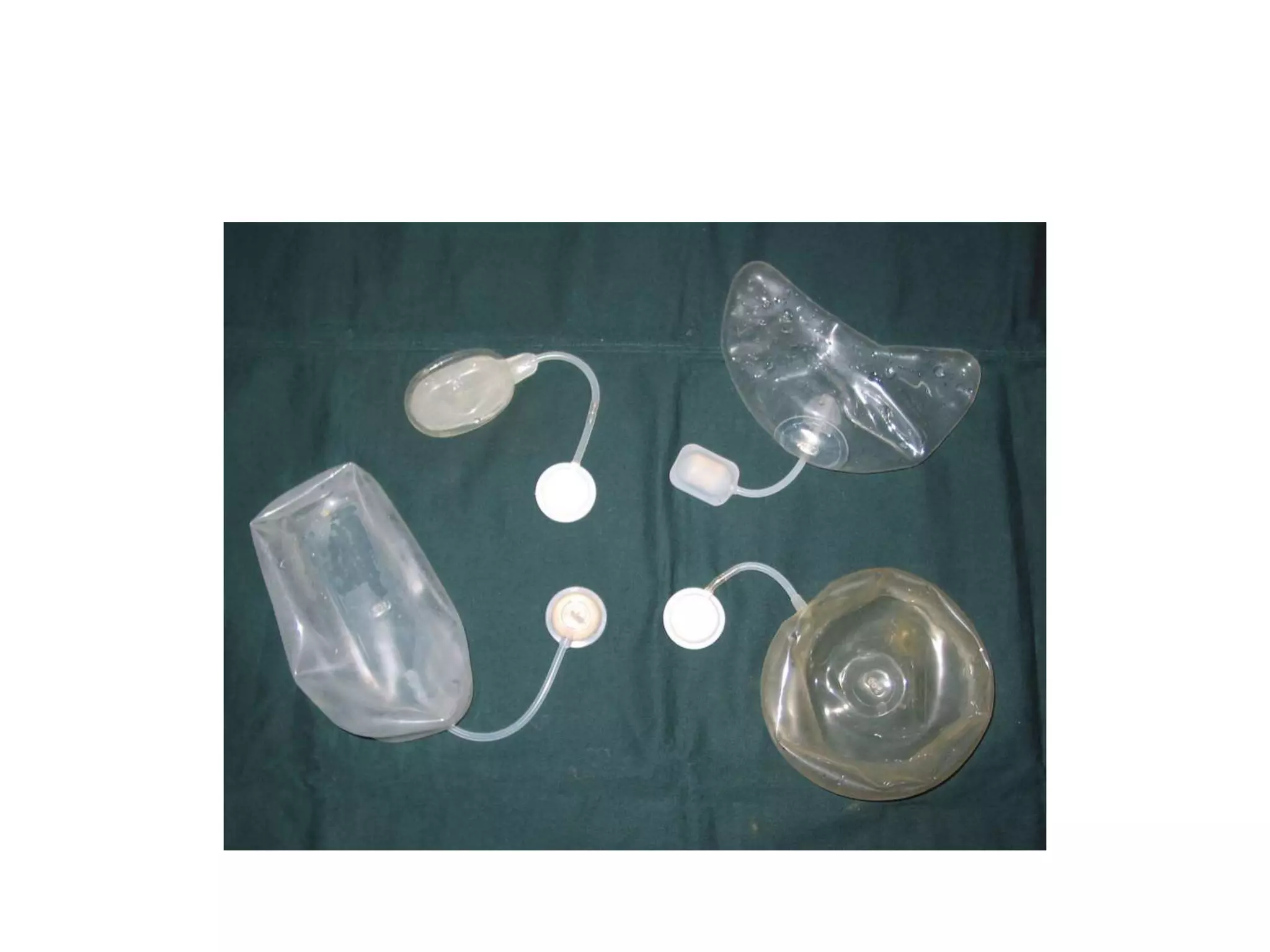
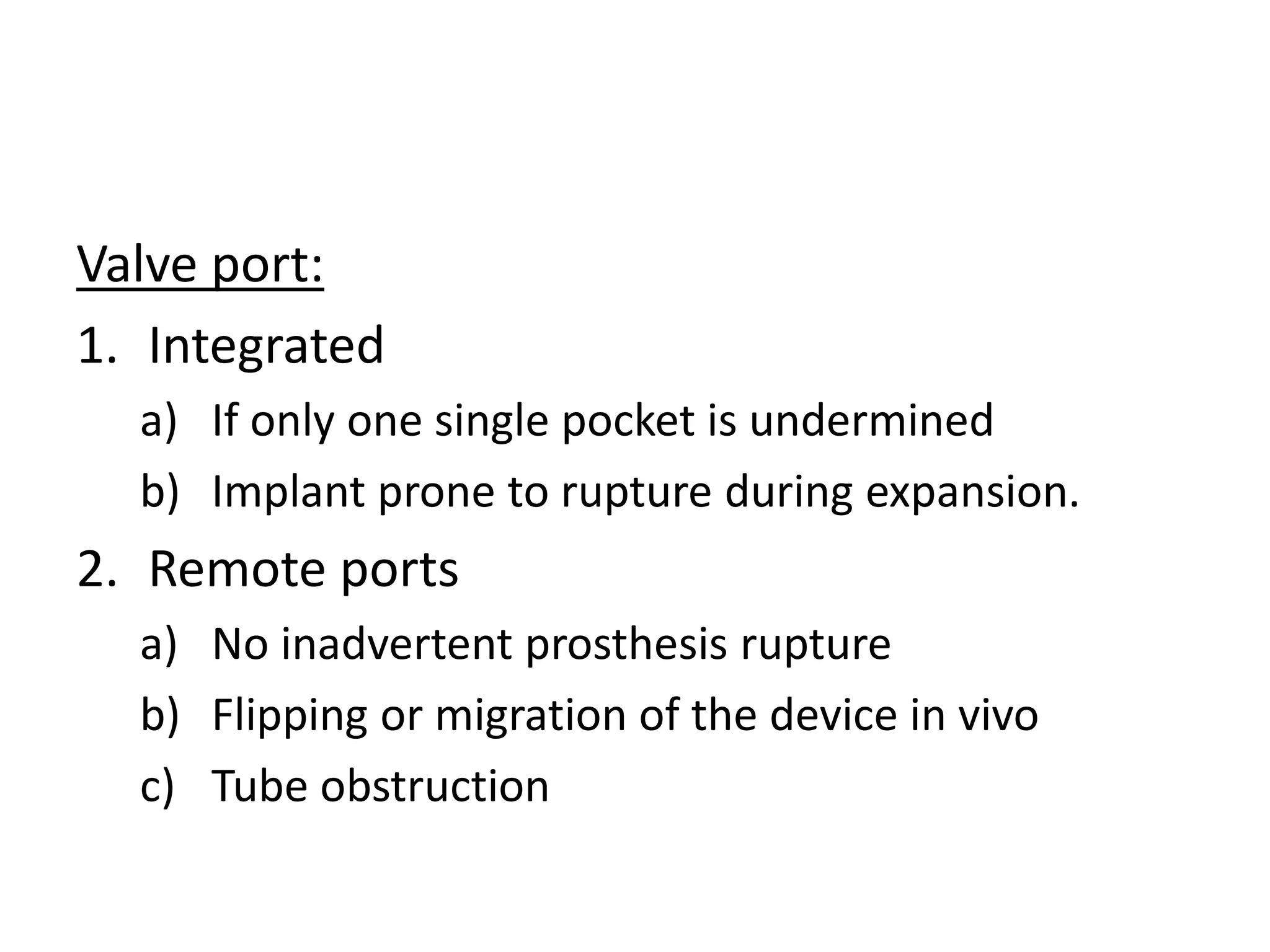
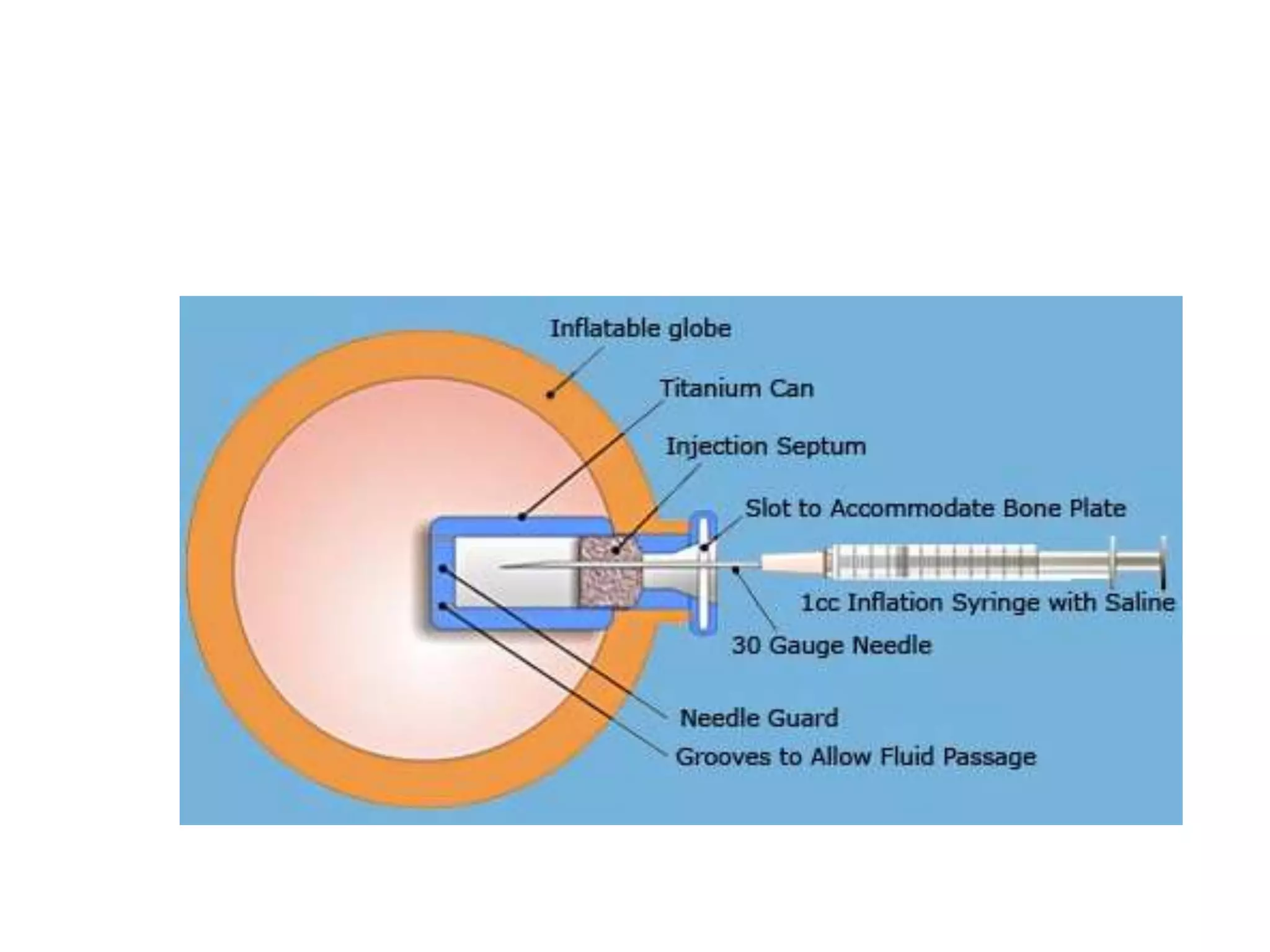
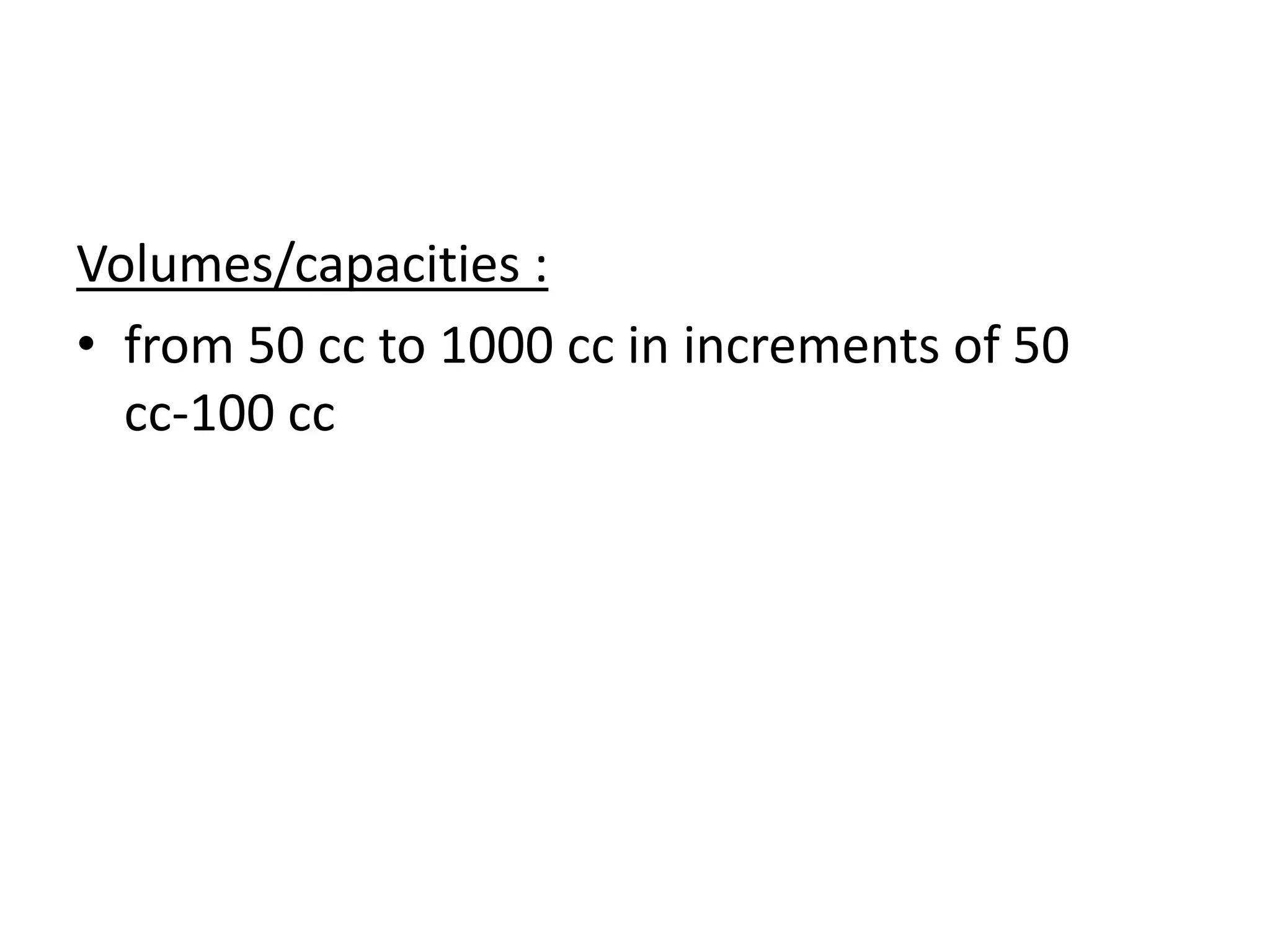
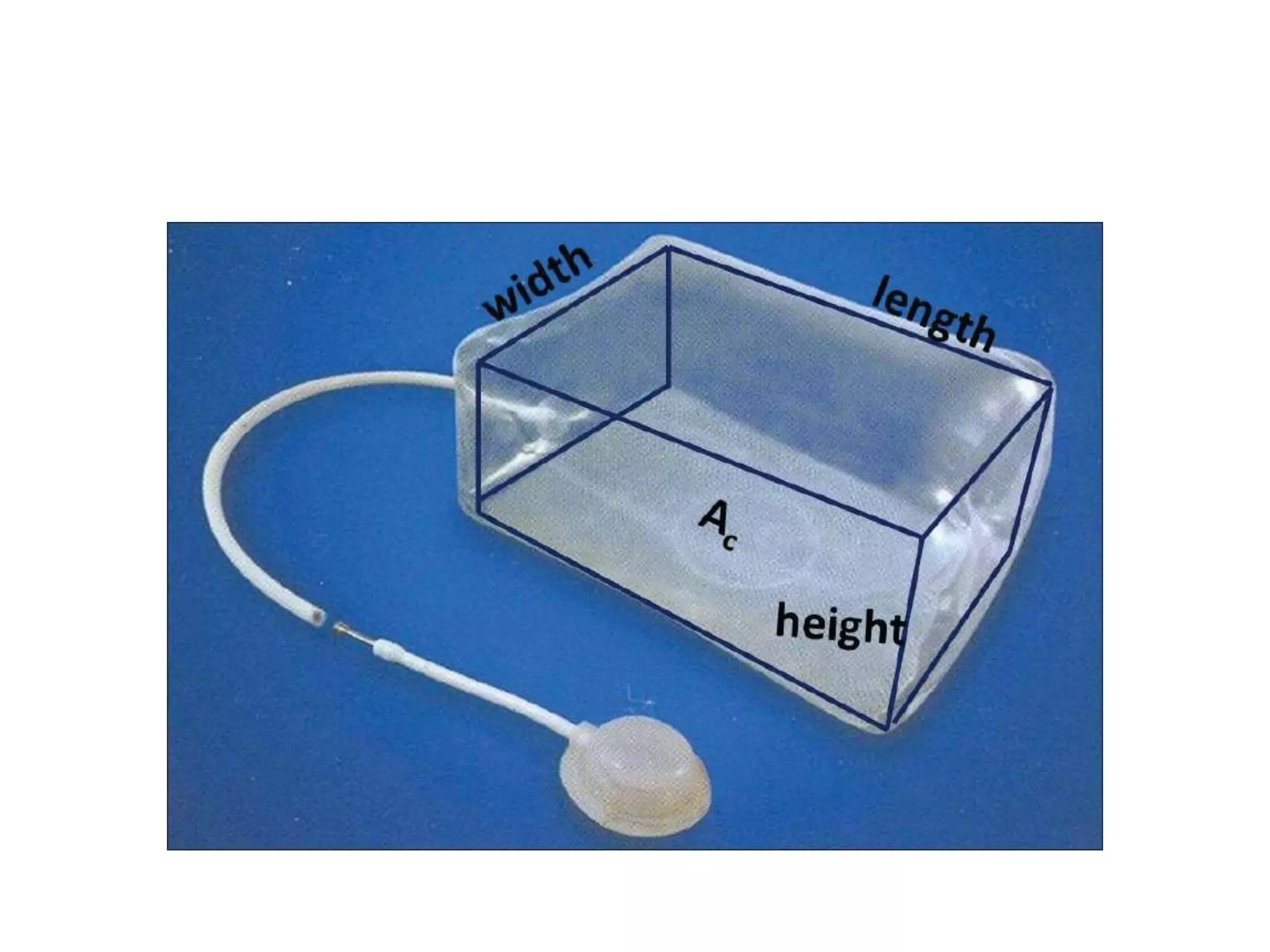
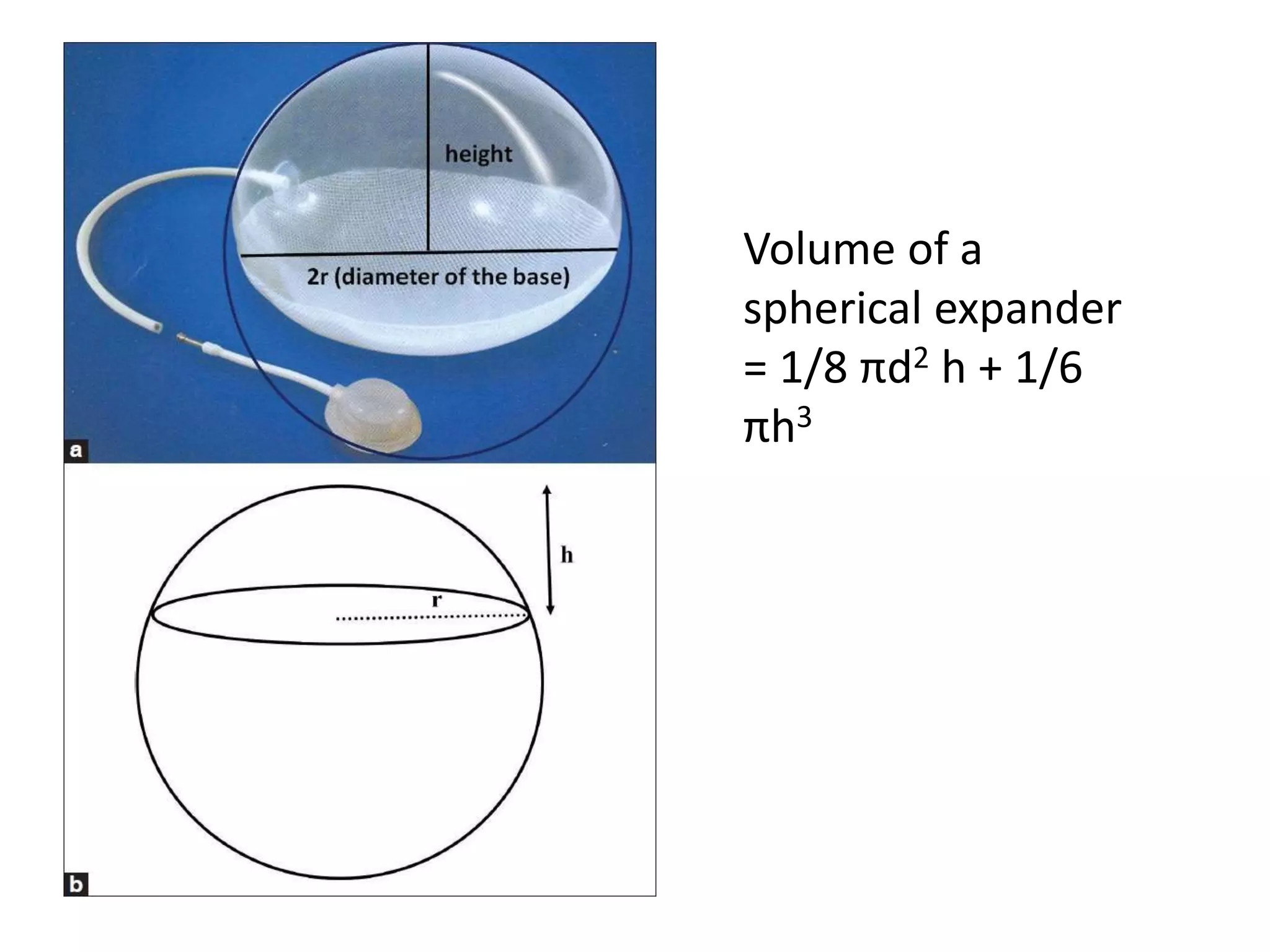
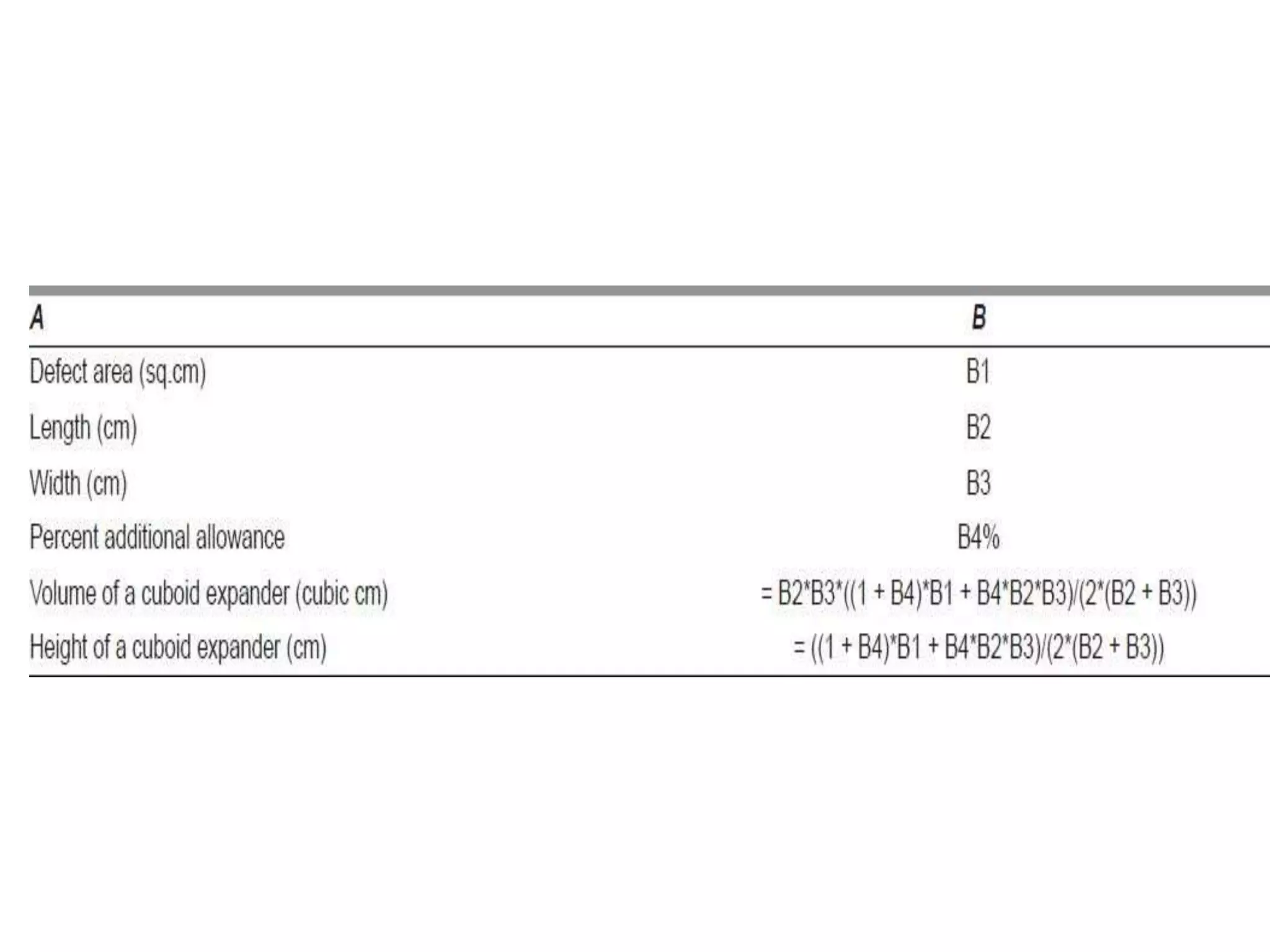
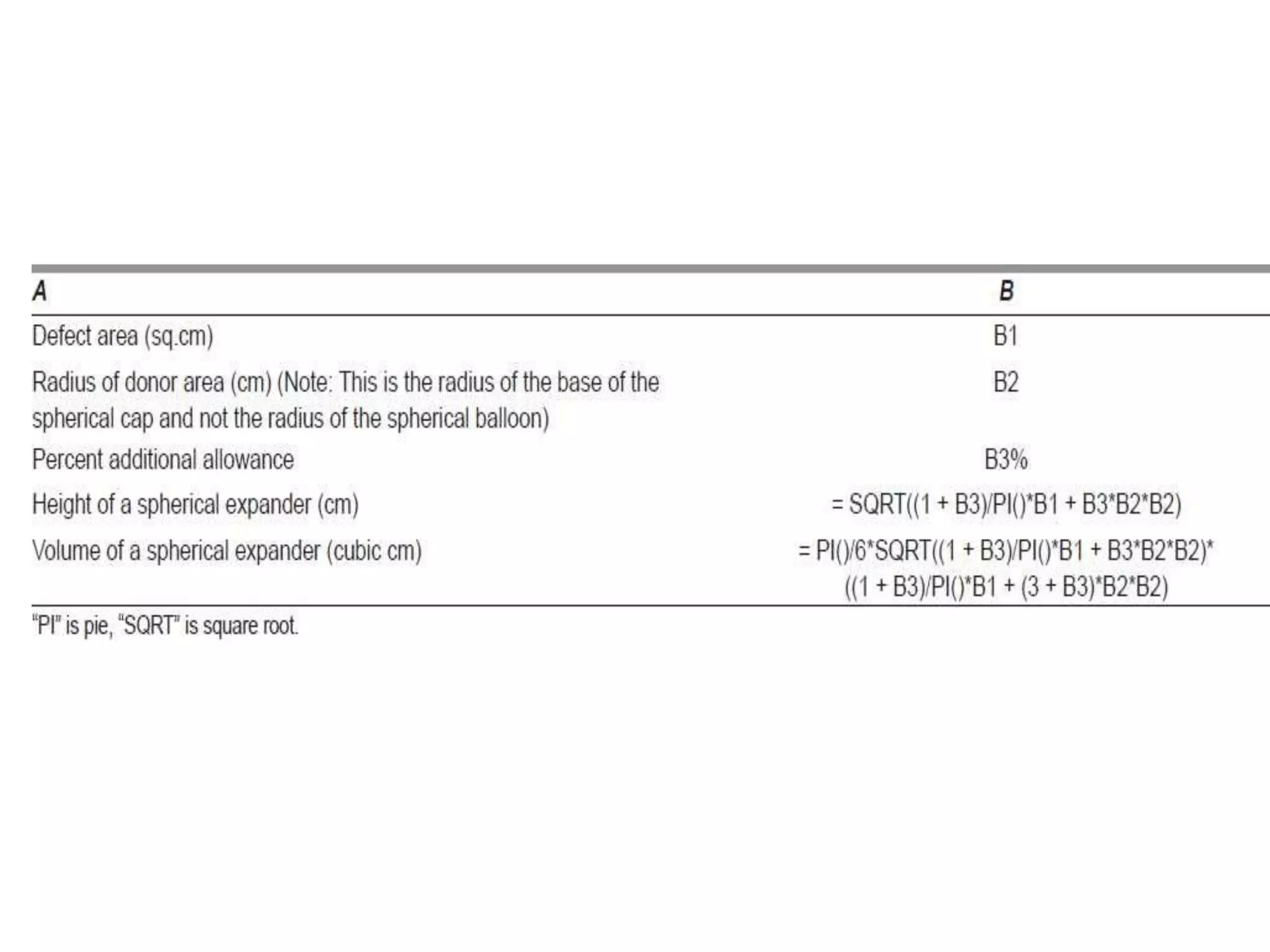
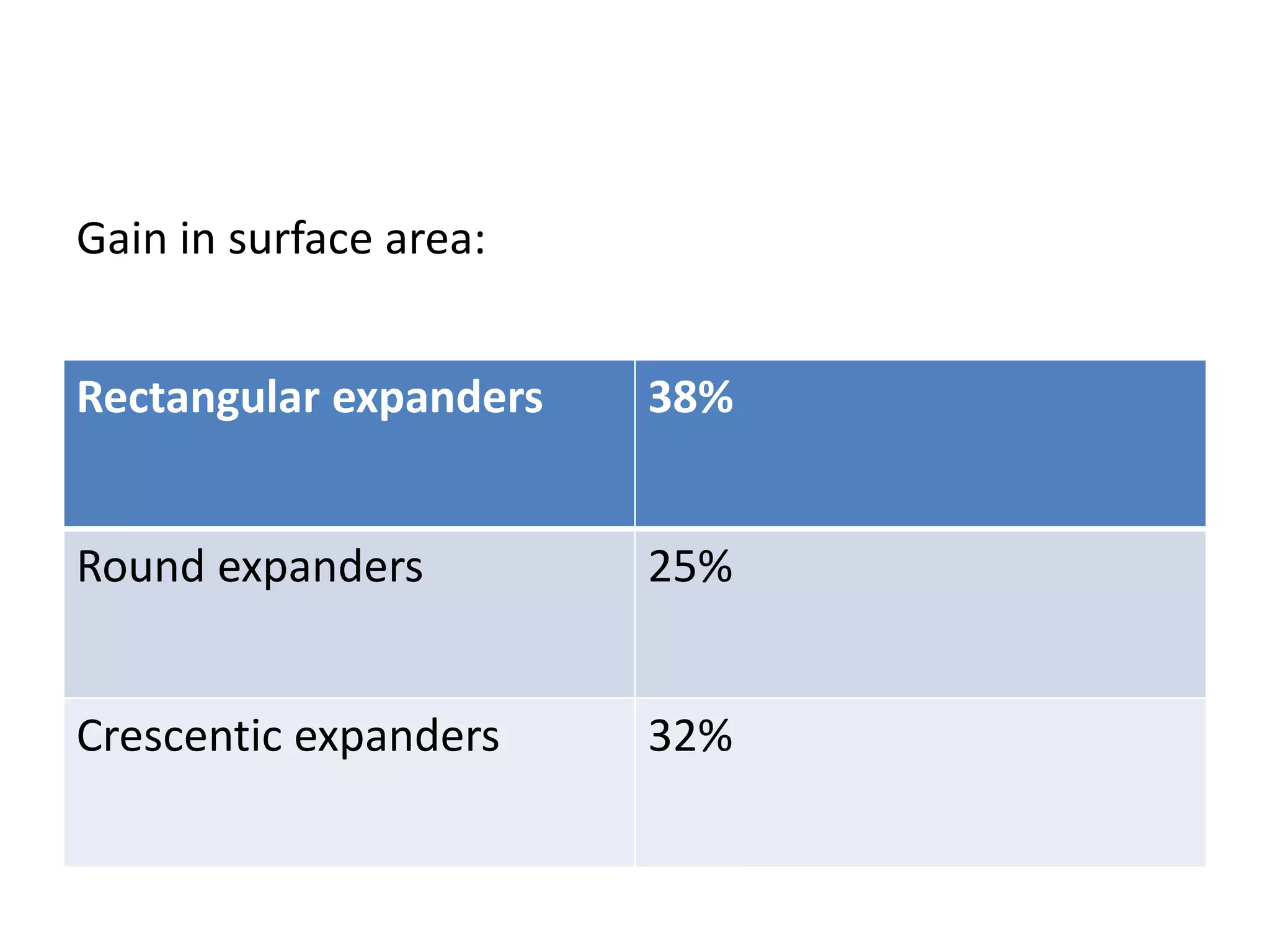
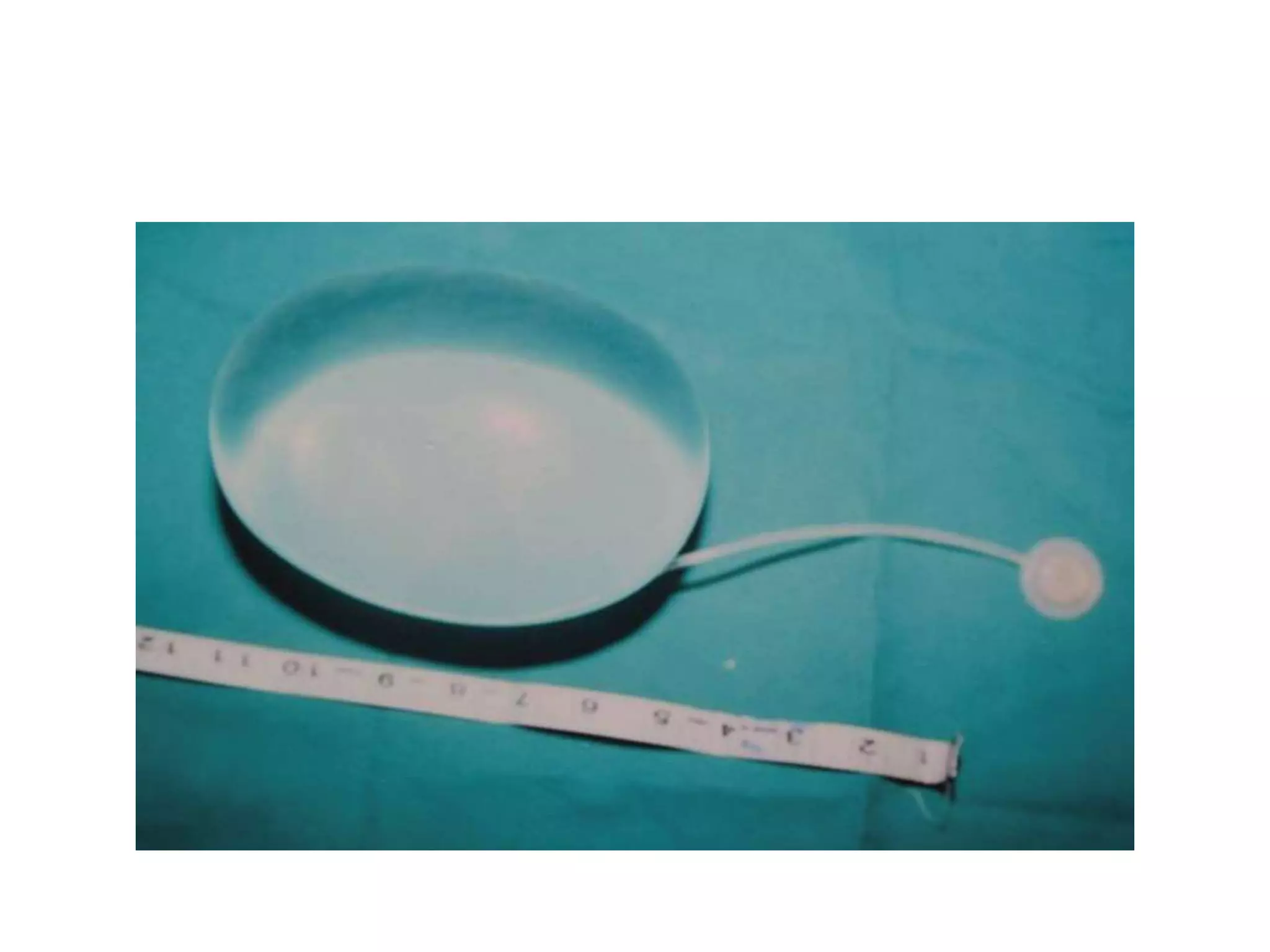
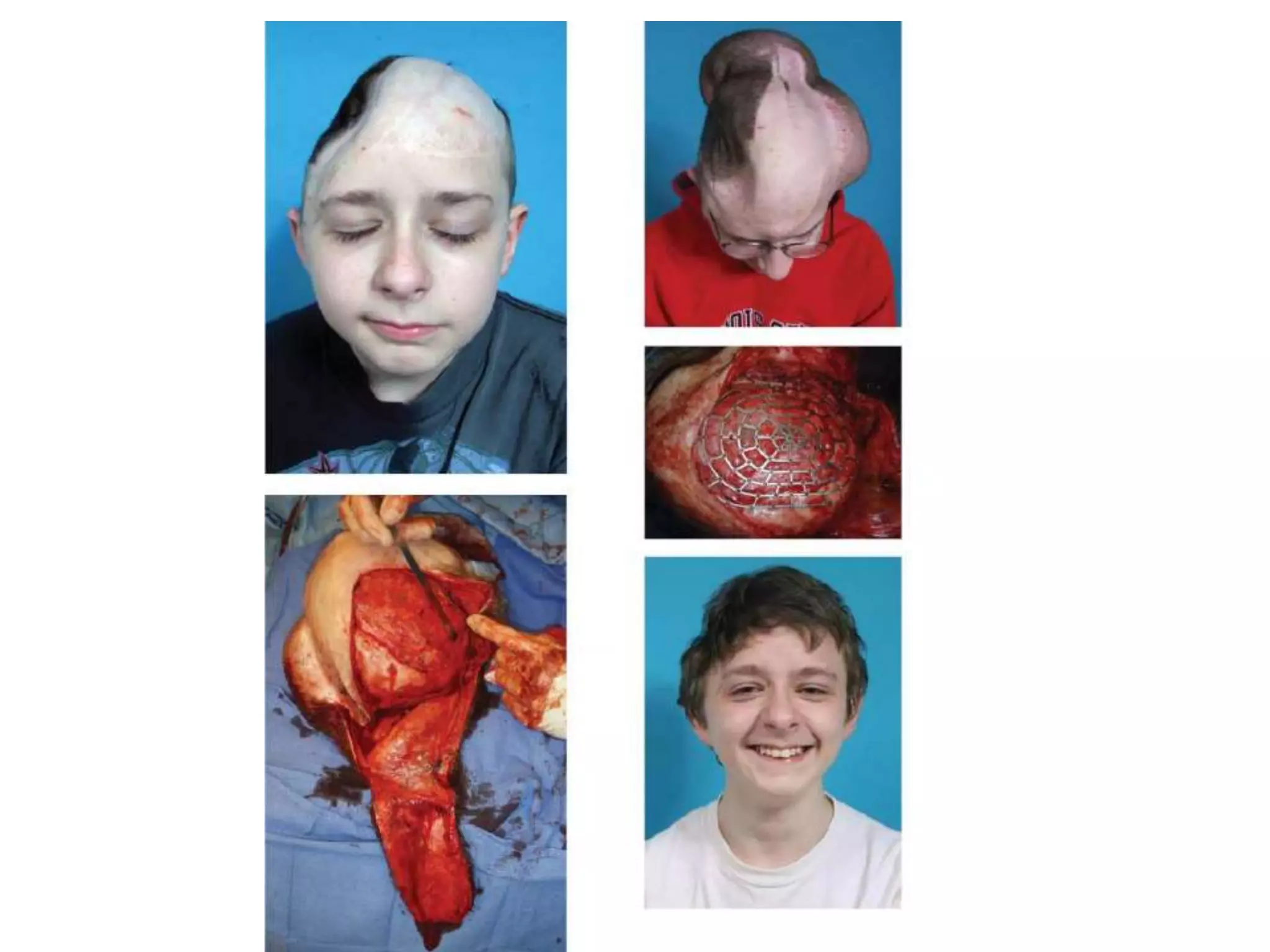
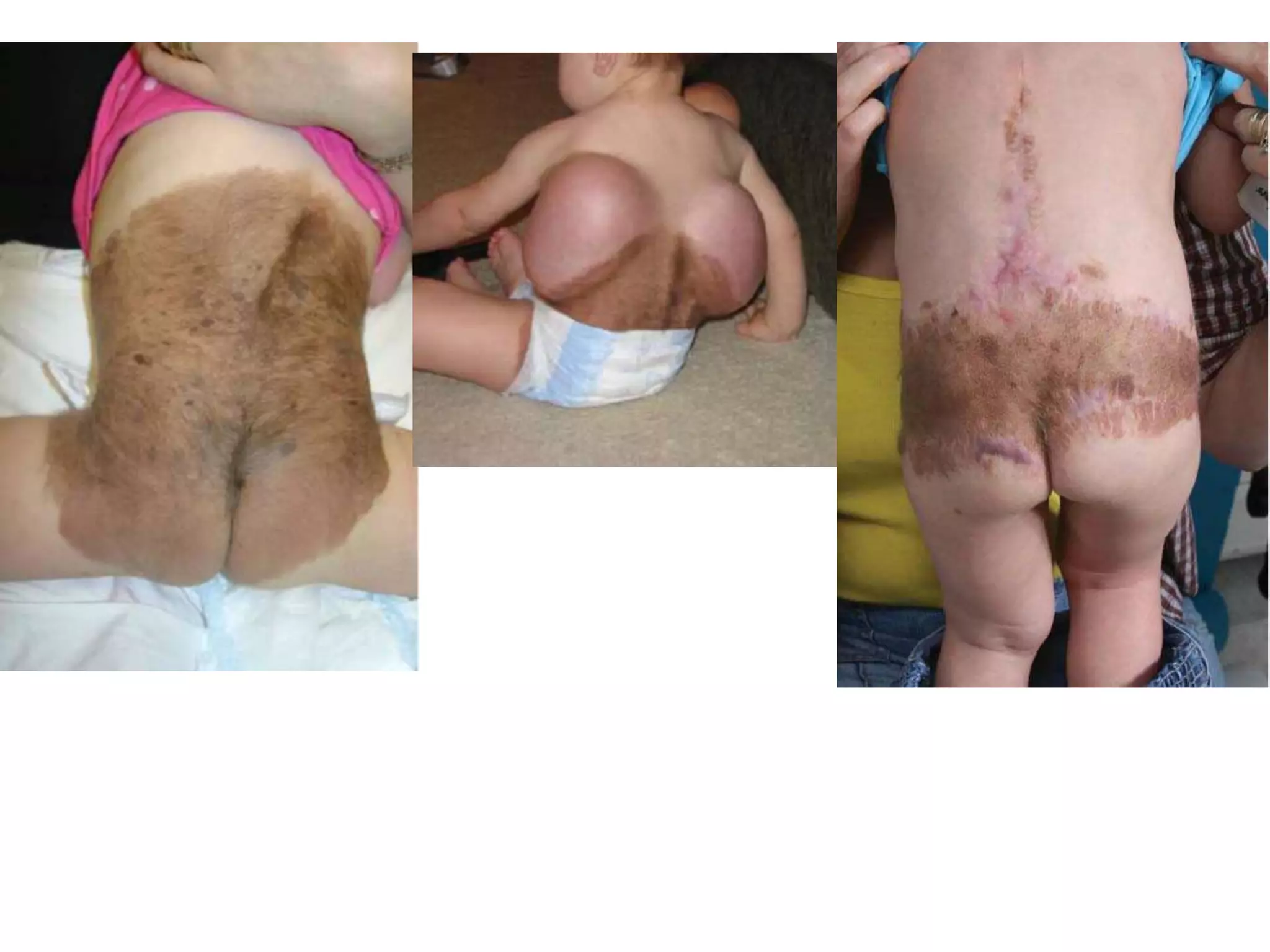
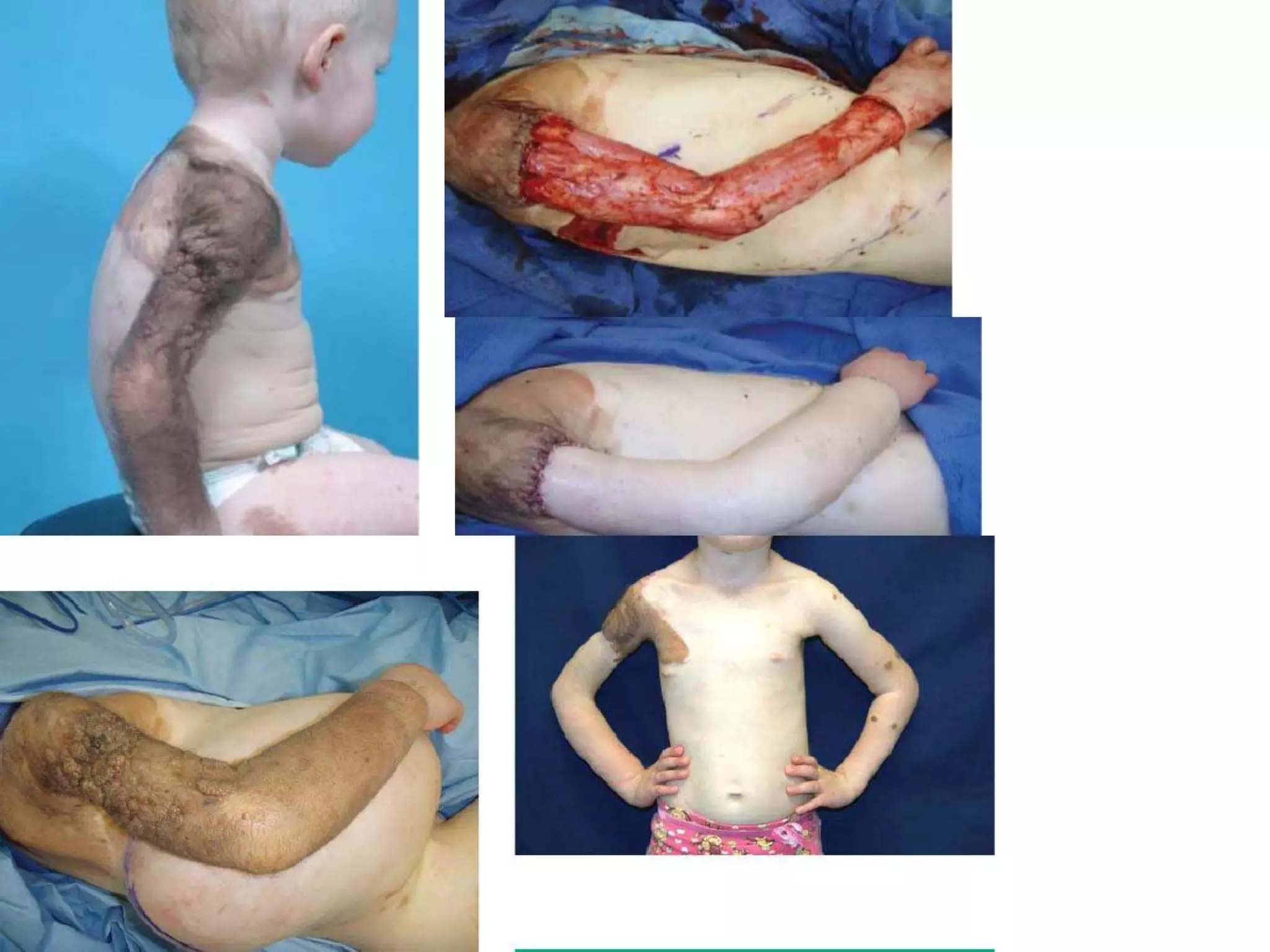
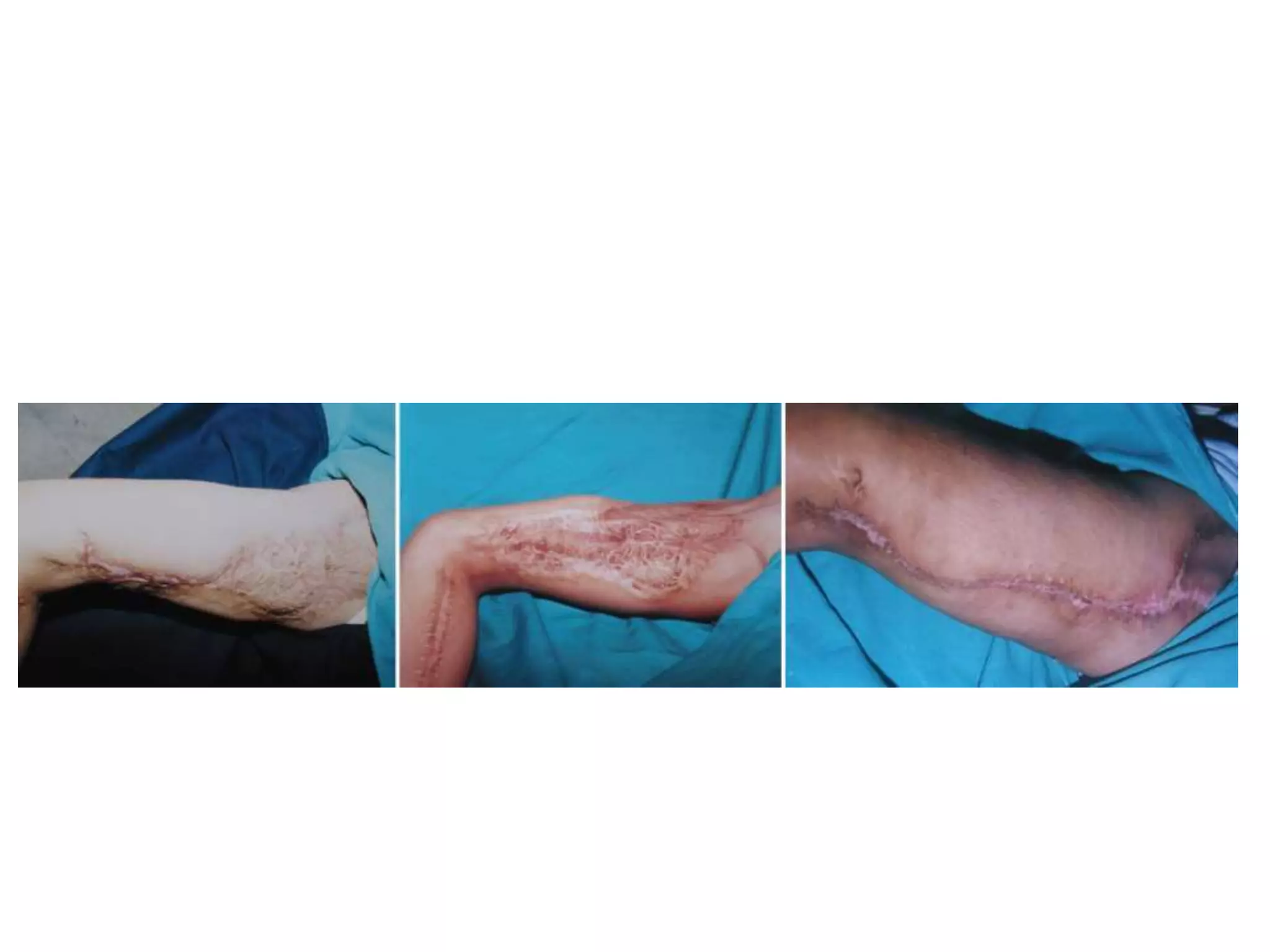
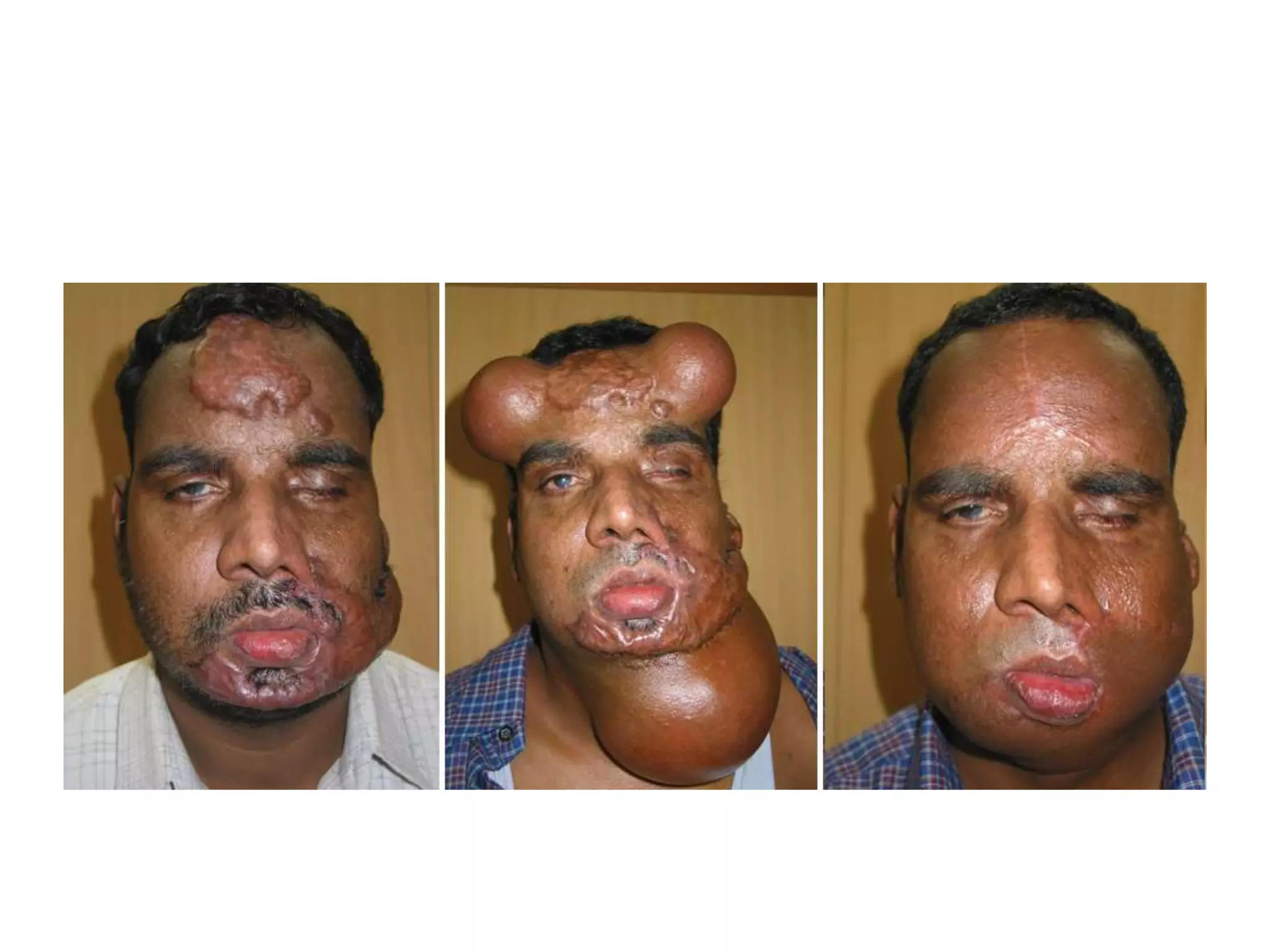
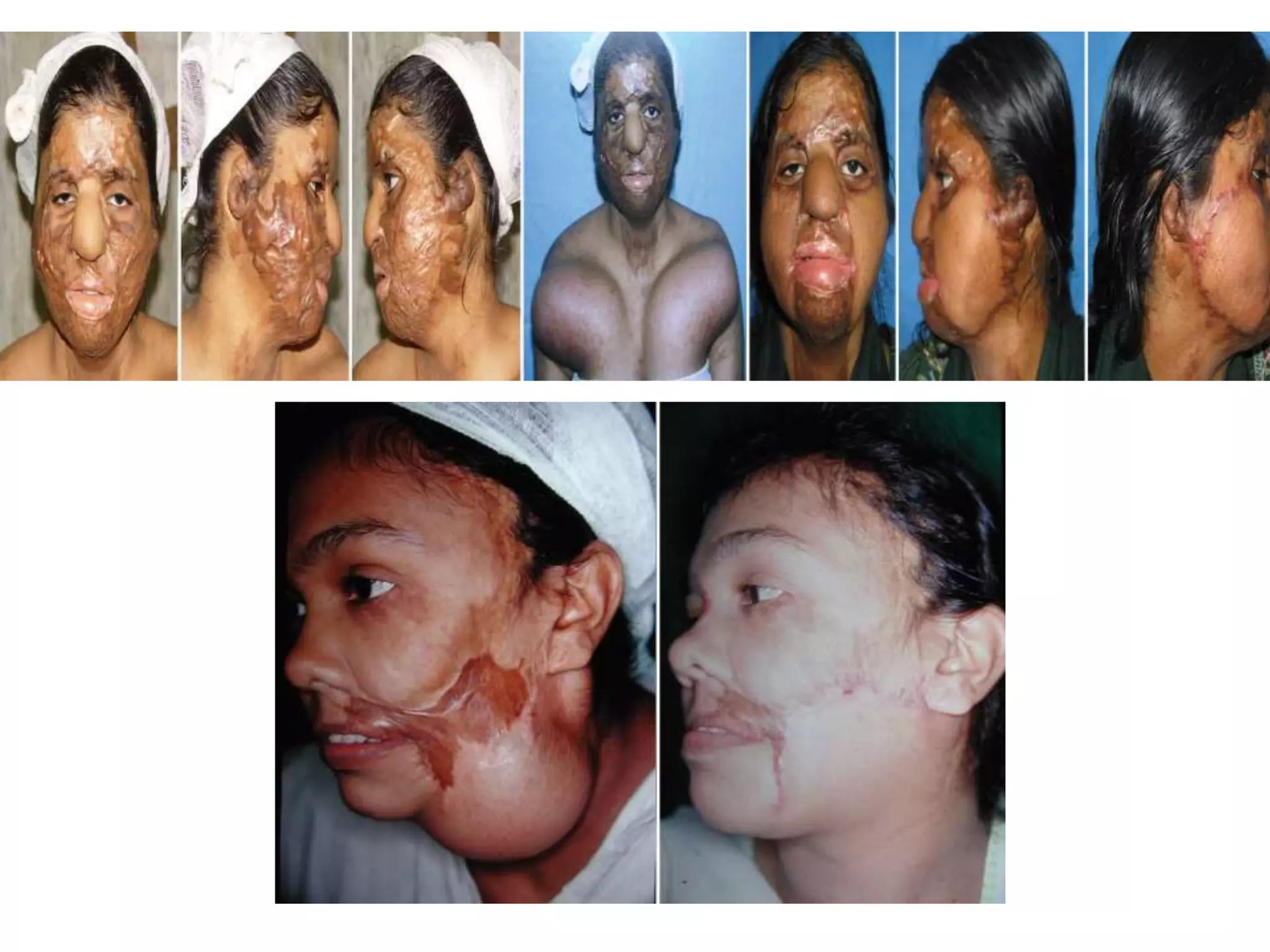
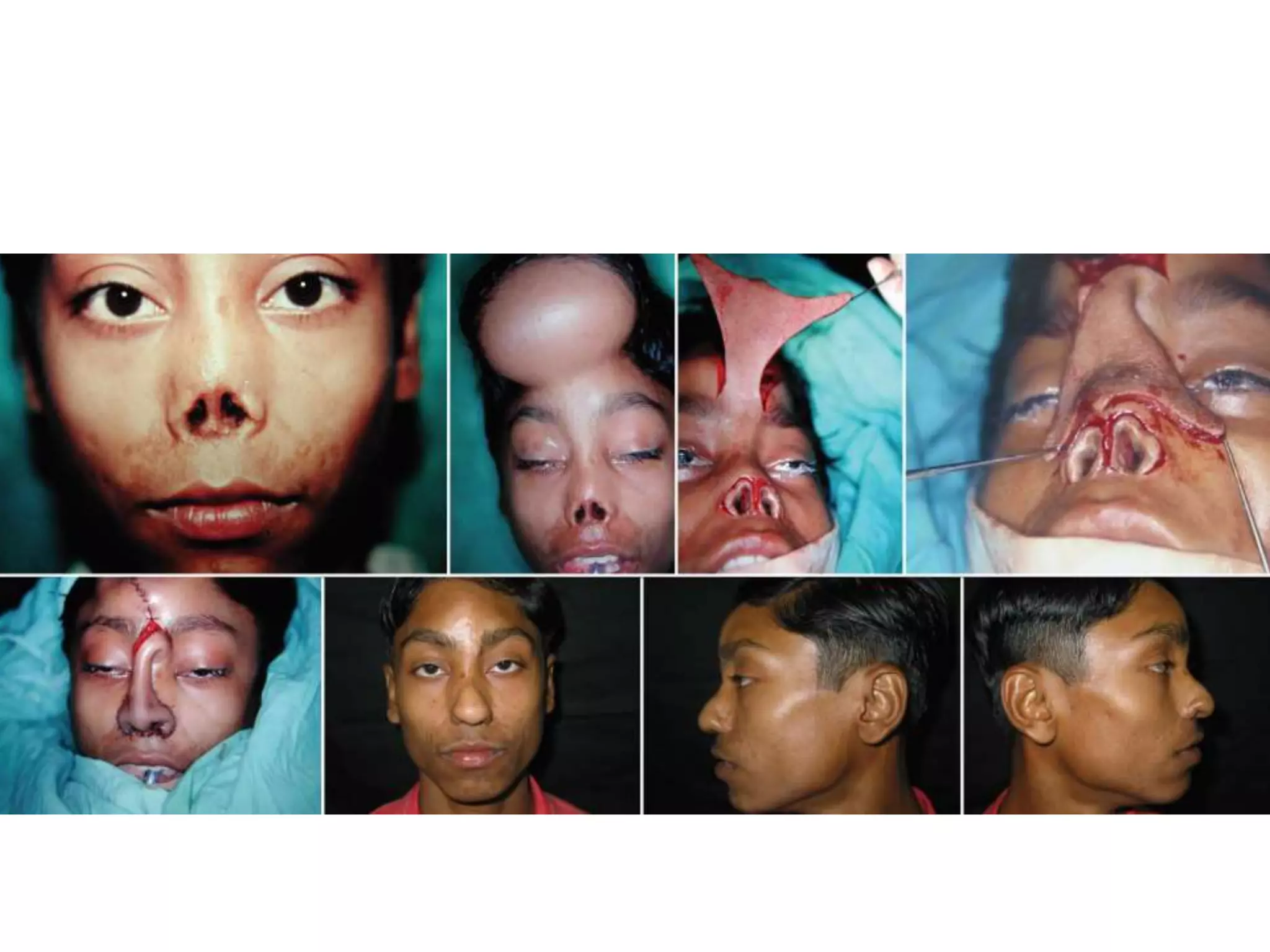
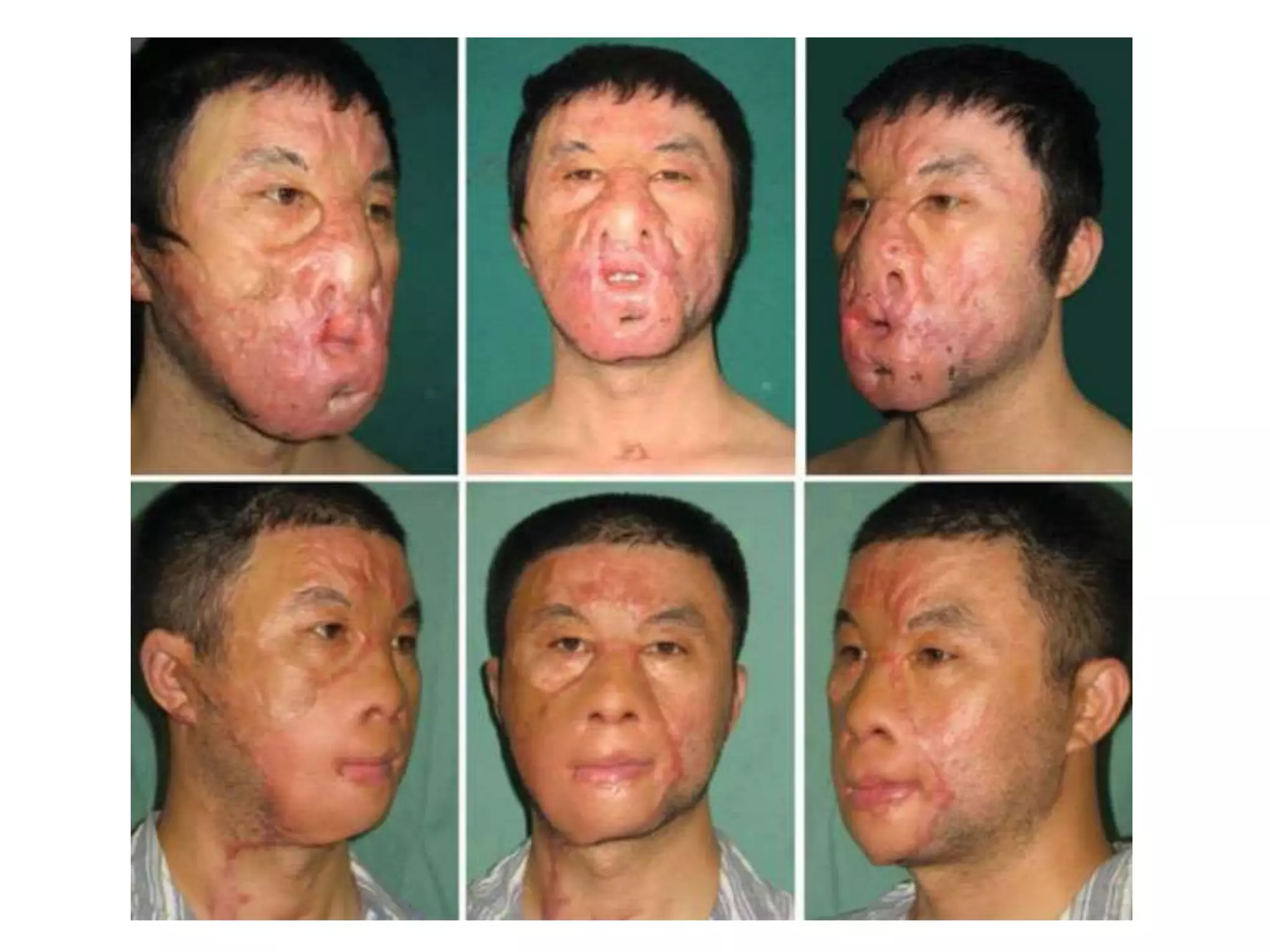
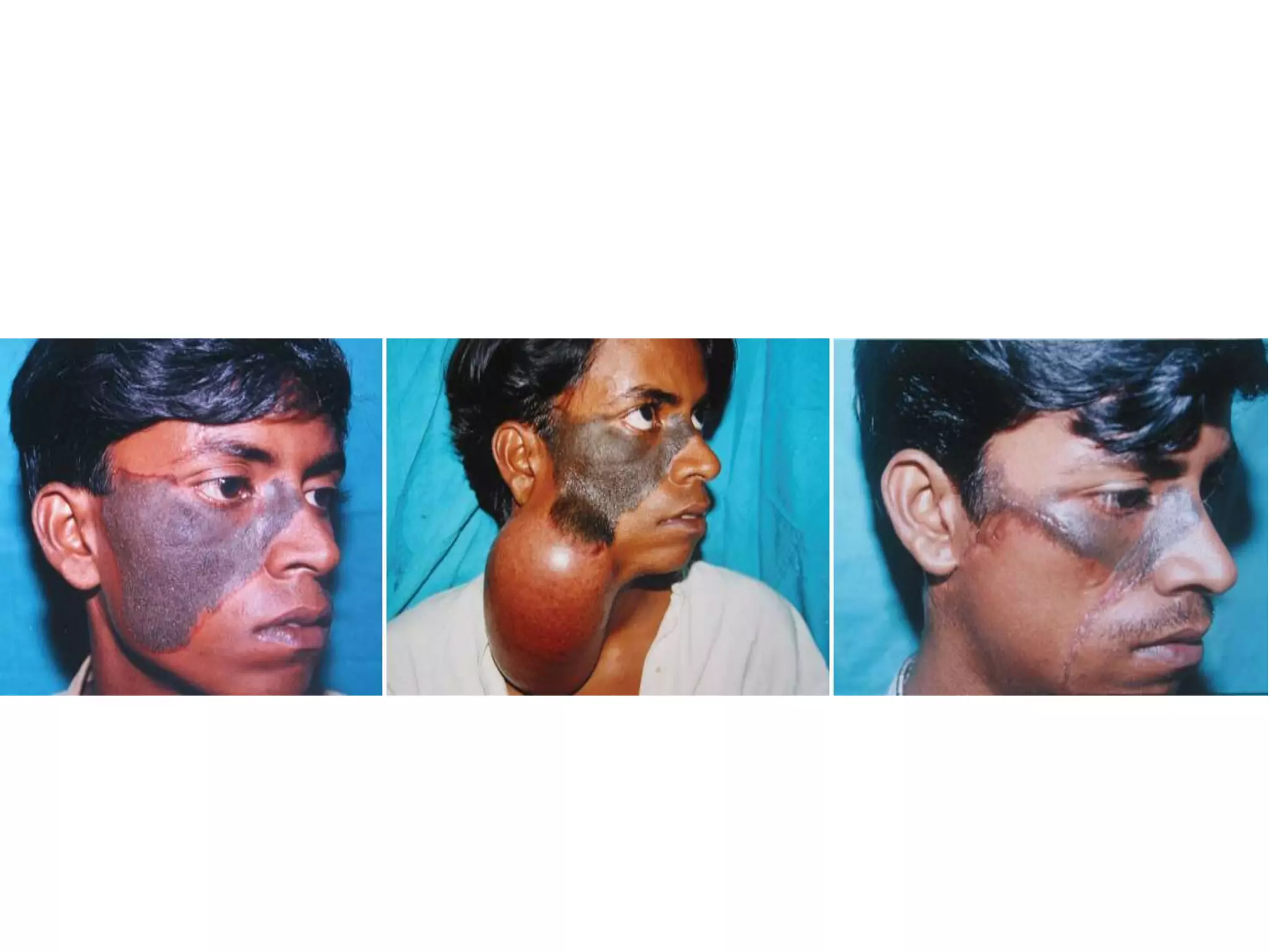
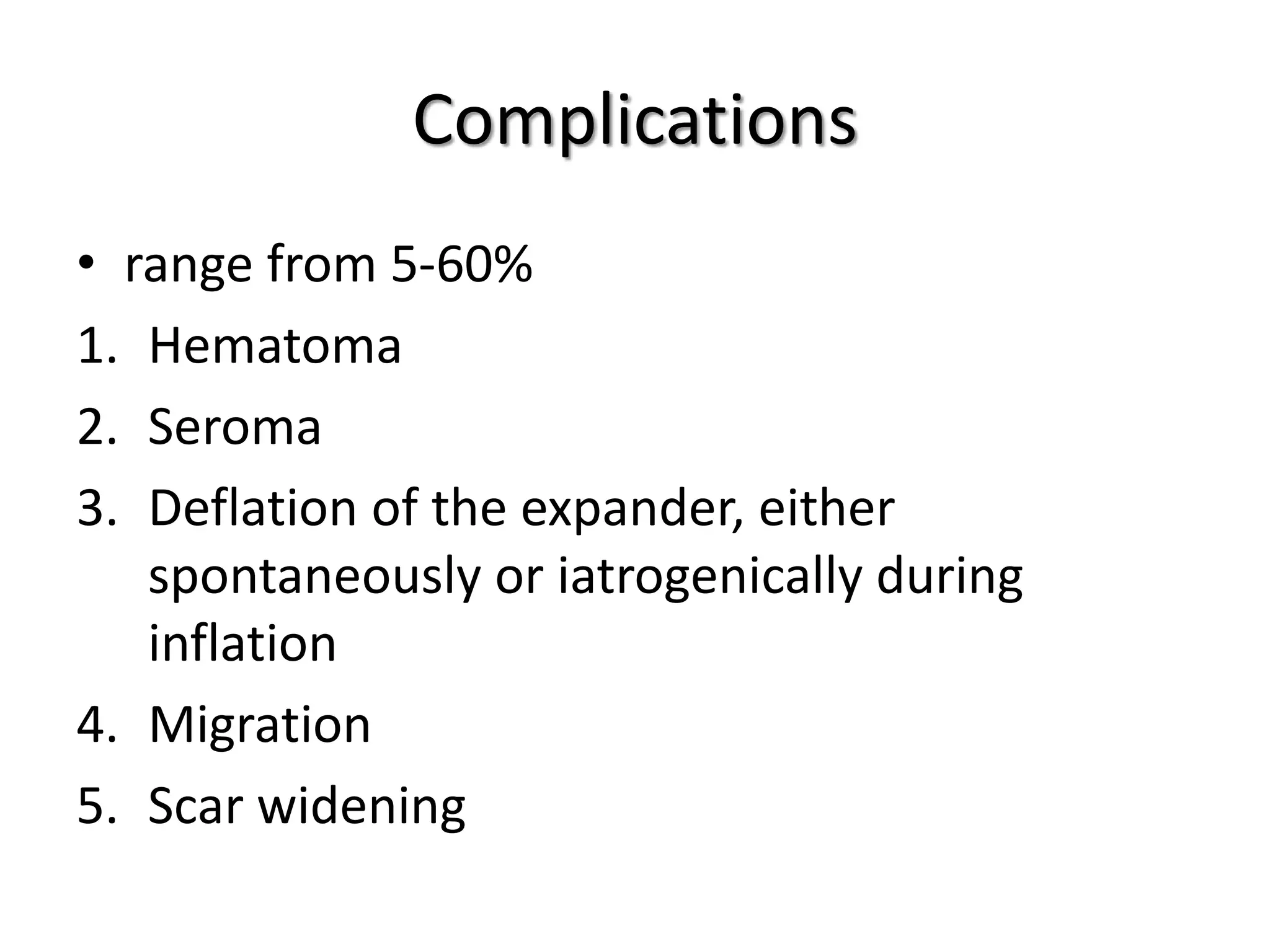
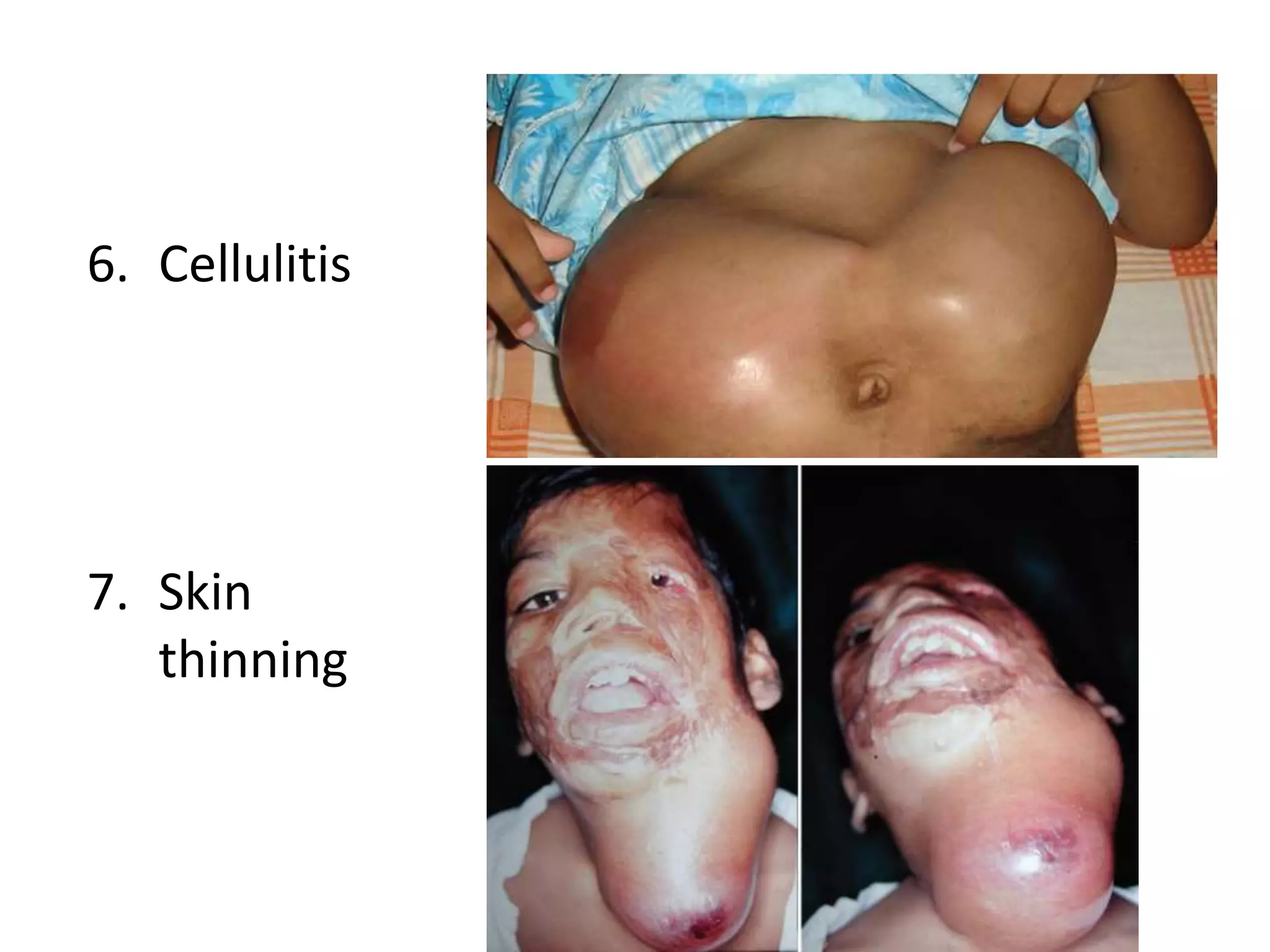
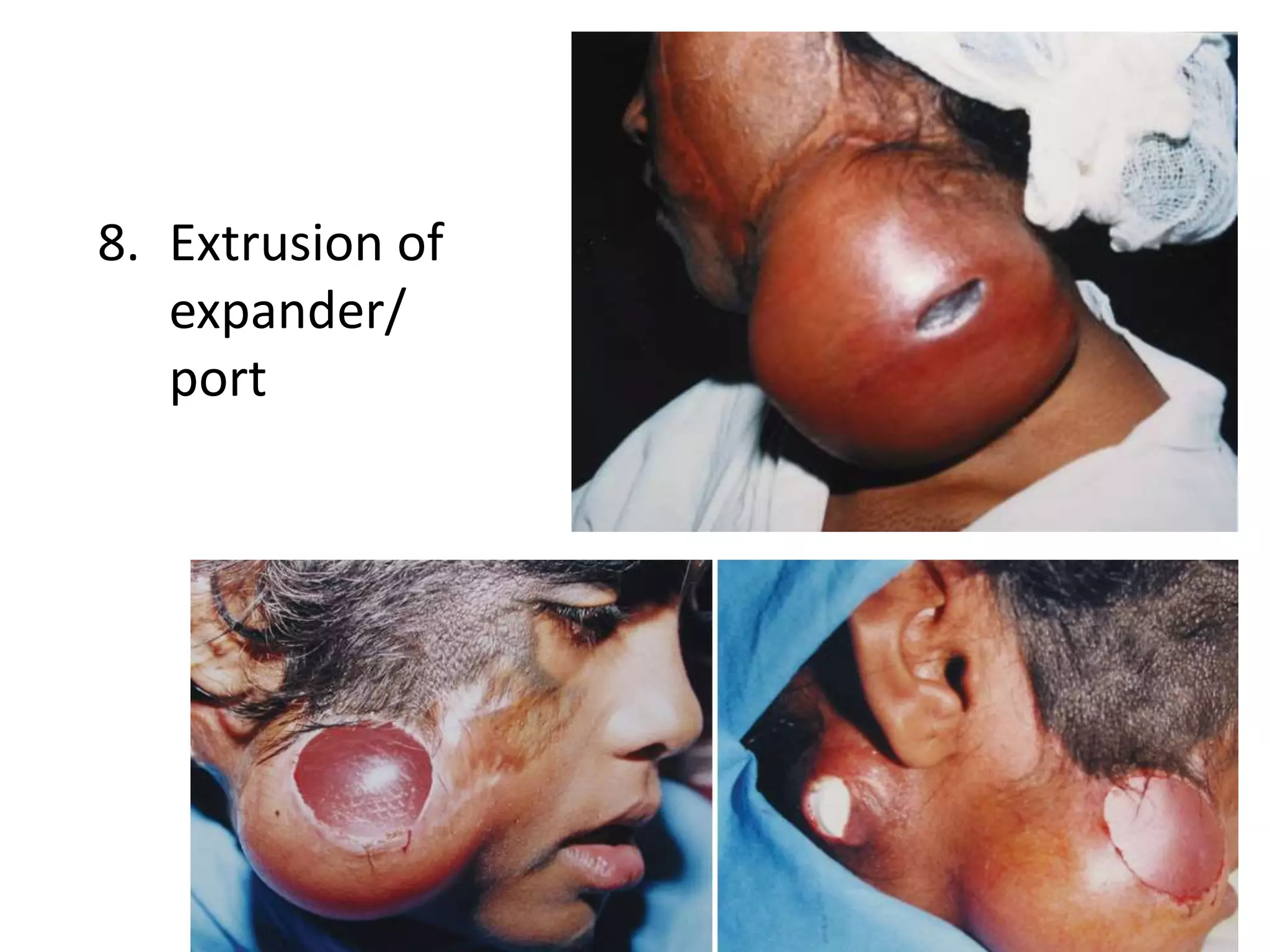
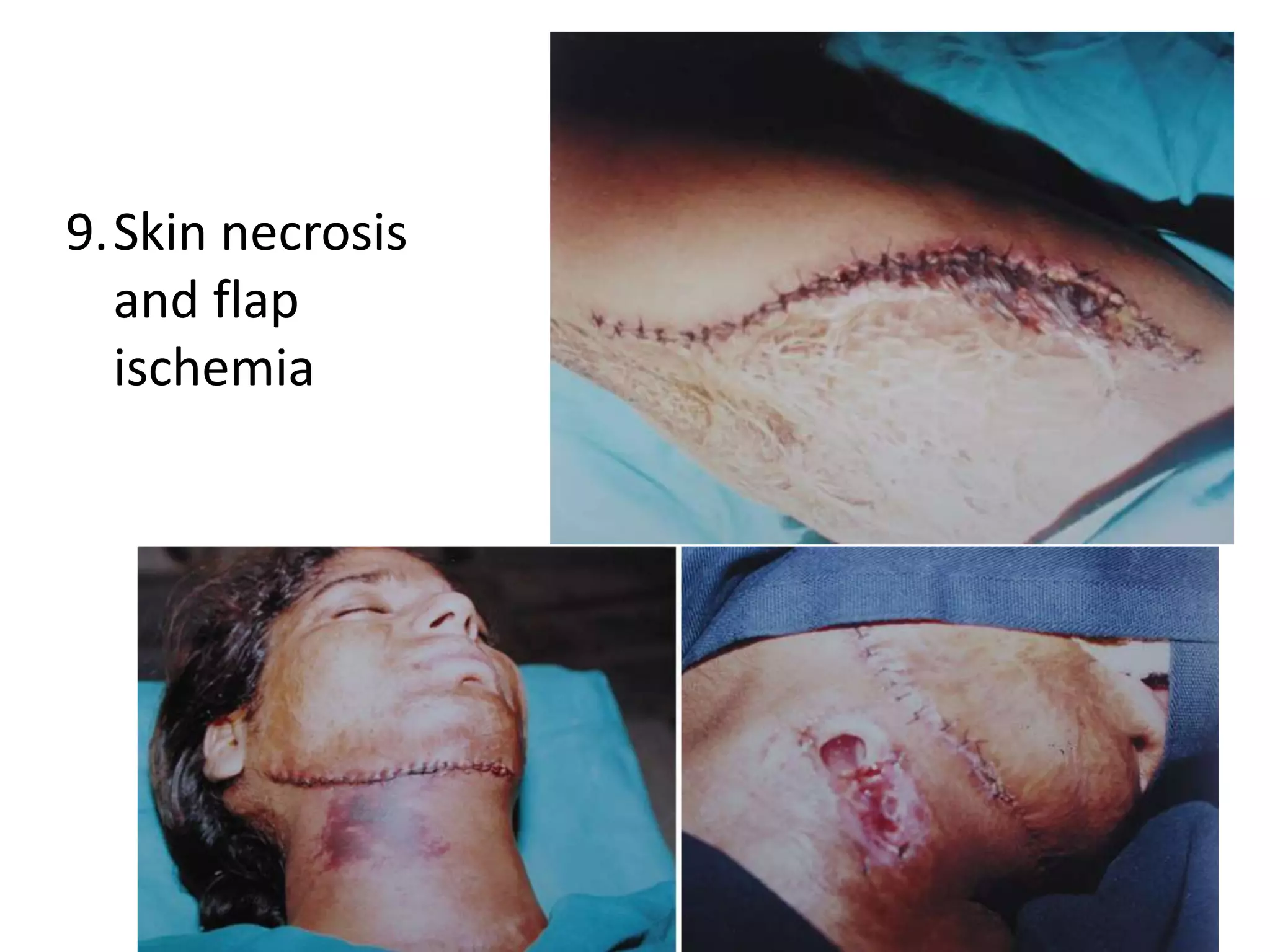
Tissue expansion is a surgical technique used to generate additional skin and soft tissue for reconstructive purposes. It involves inserting a temporary implant called a tissue expander under the skin and gradually inflating it with saline over 6-12 weeks to stretch the overlying tissue. This causes mechanical and biological tissue growth. The expander is then removed and the expanded skin is advanced to reconstruct areas of skin loss or defects. Complications can include hematoma, seroma, expander deflation or migration, and skin thinning or necrosis but tissue expansion provides a good source of autologous tissue for reconstruction when other options are limited.