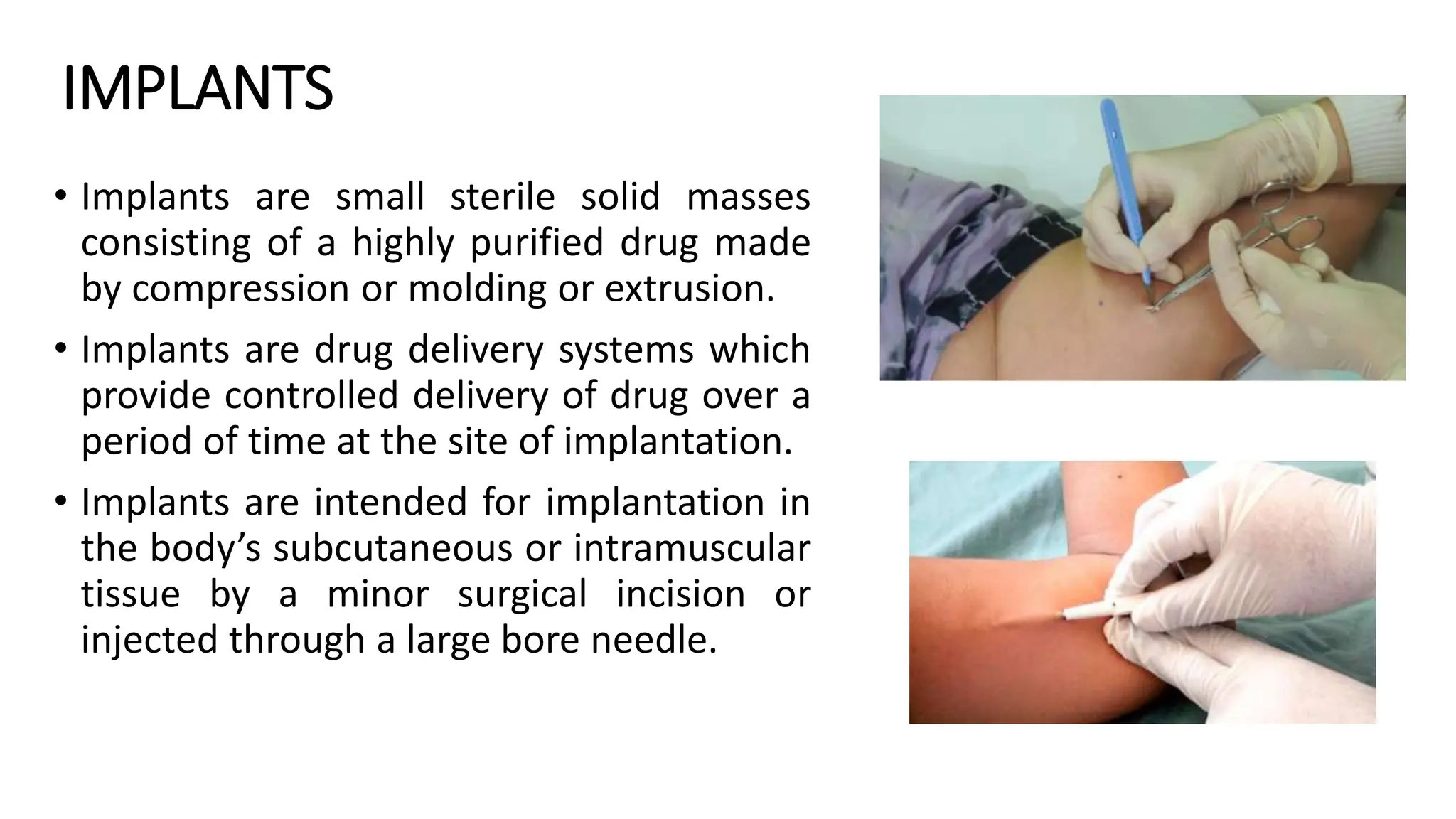
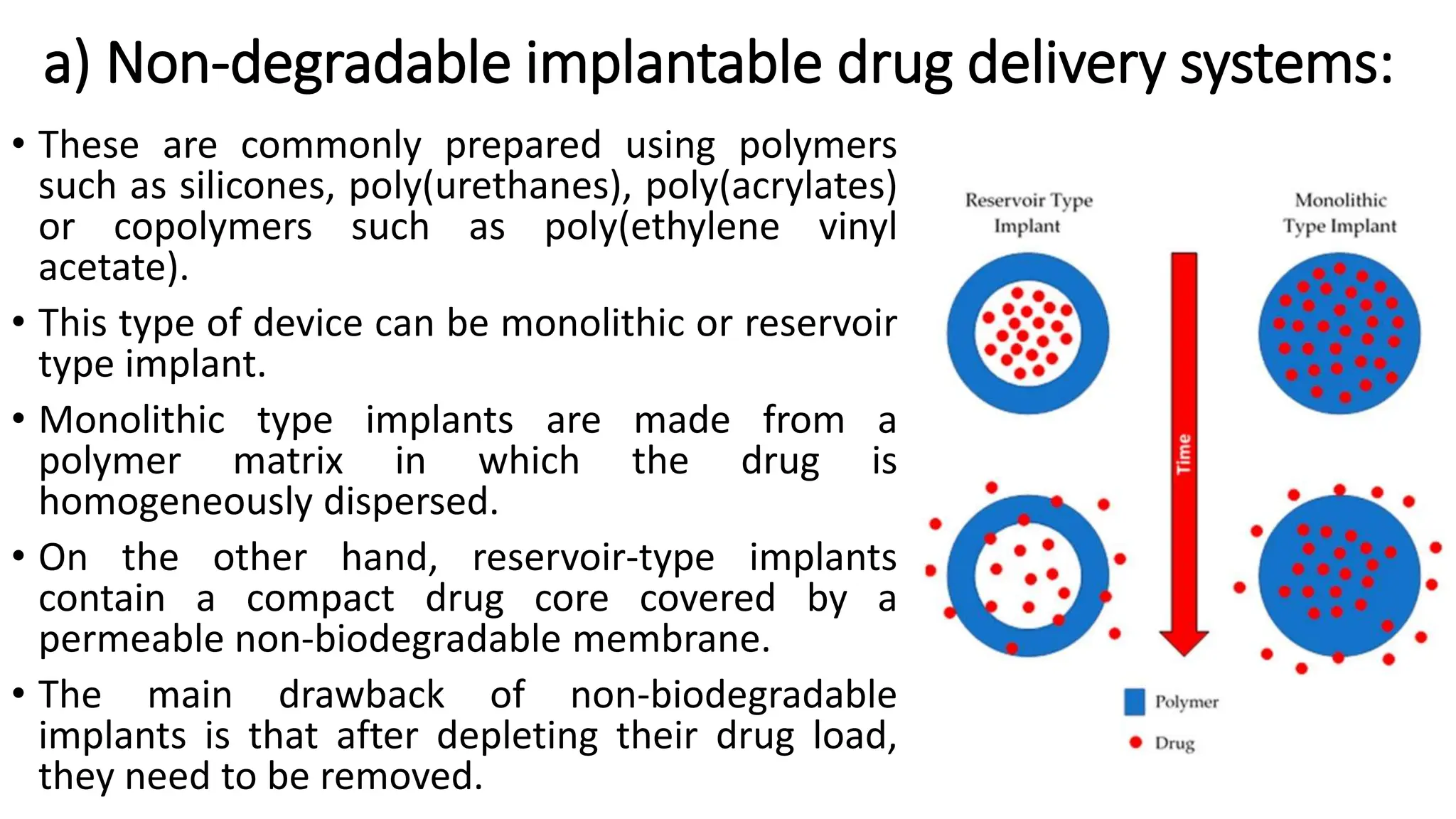
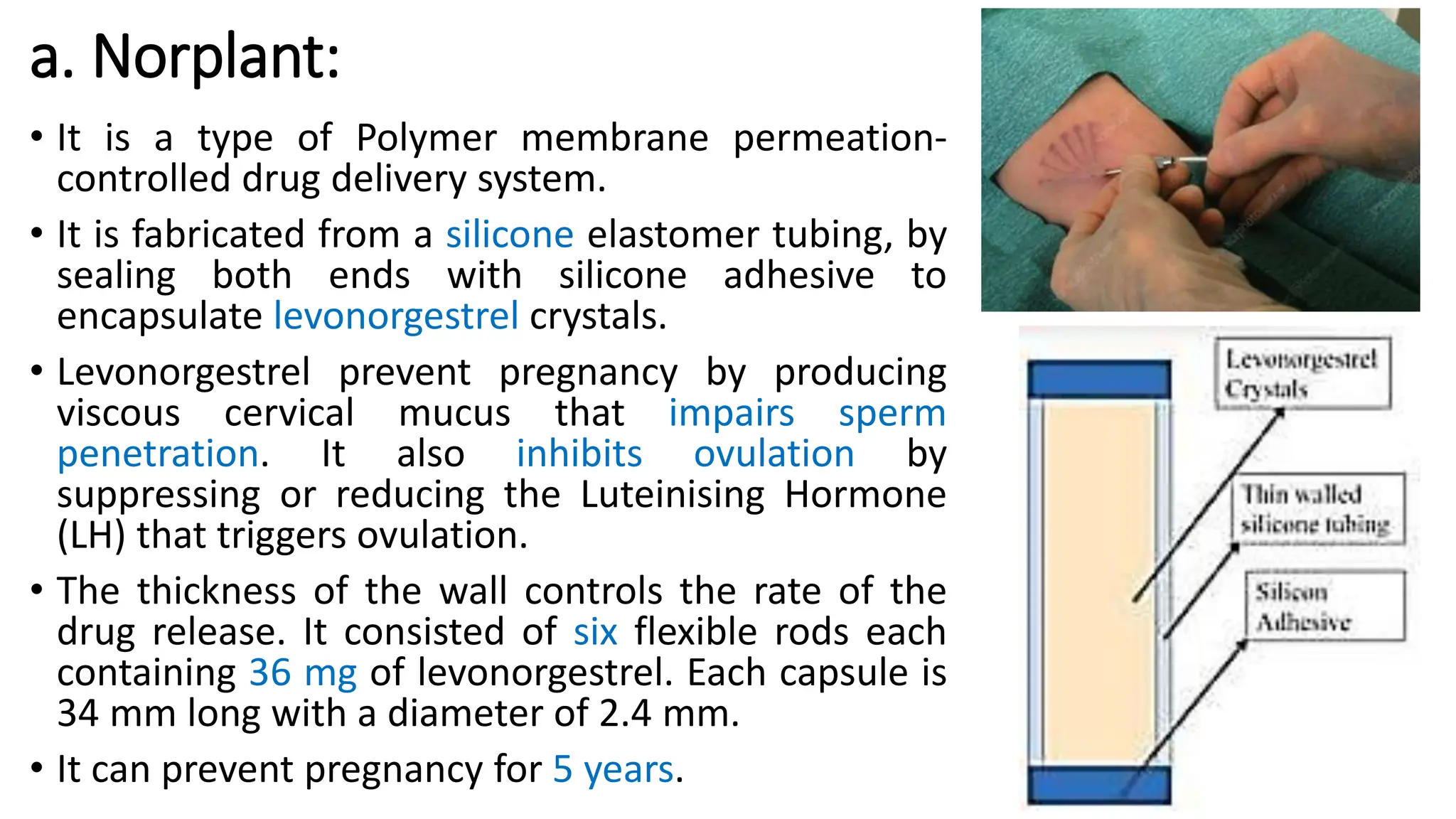
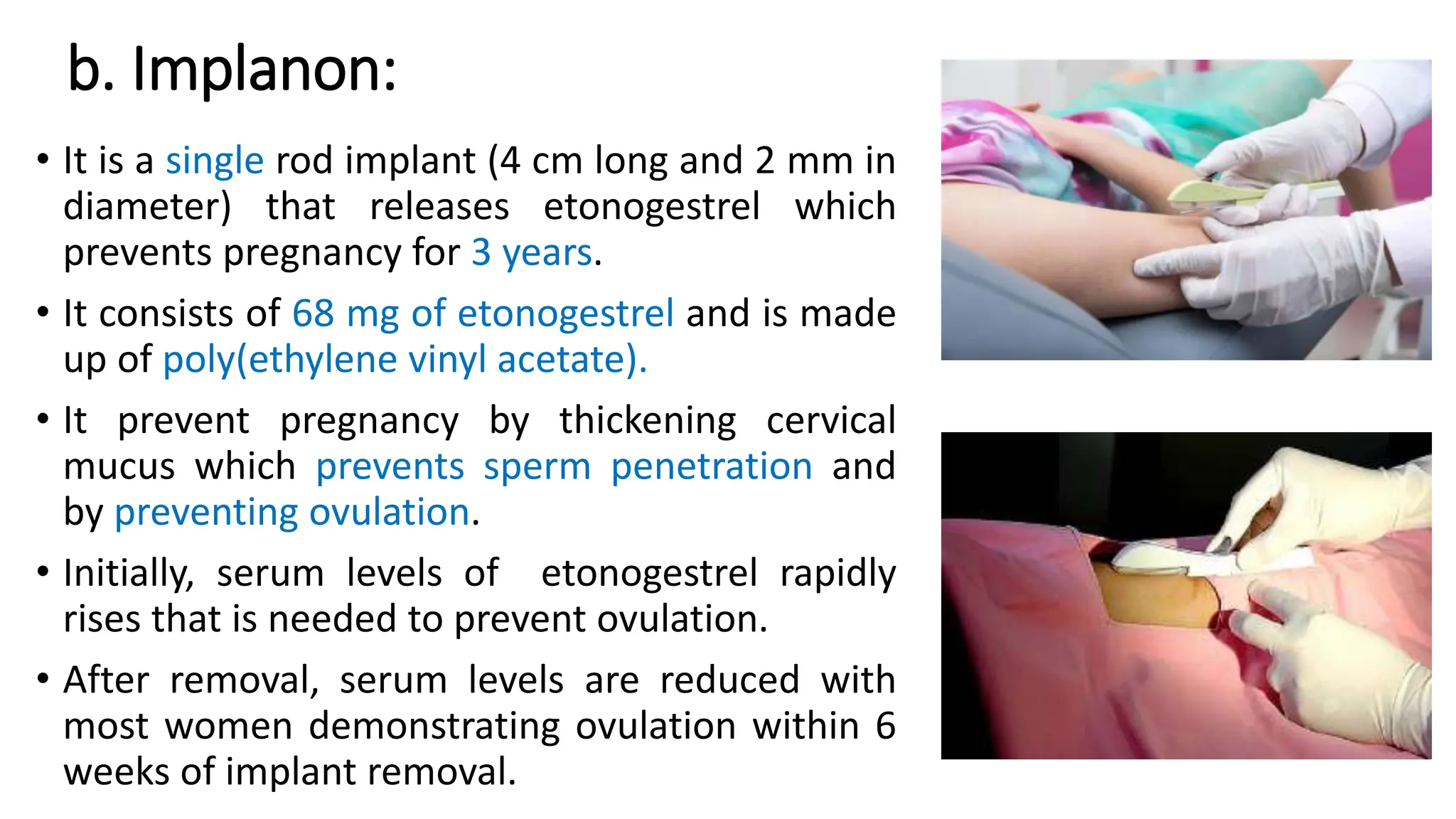
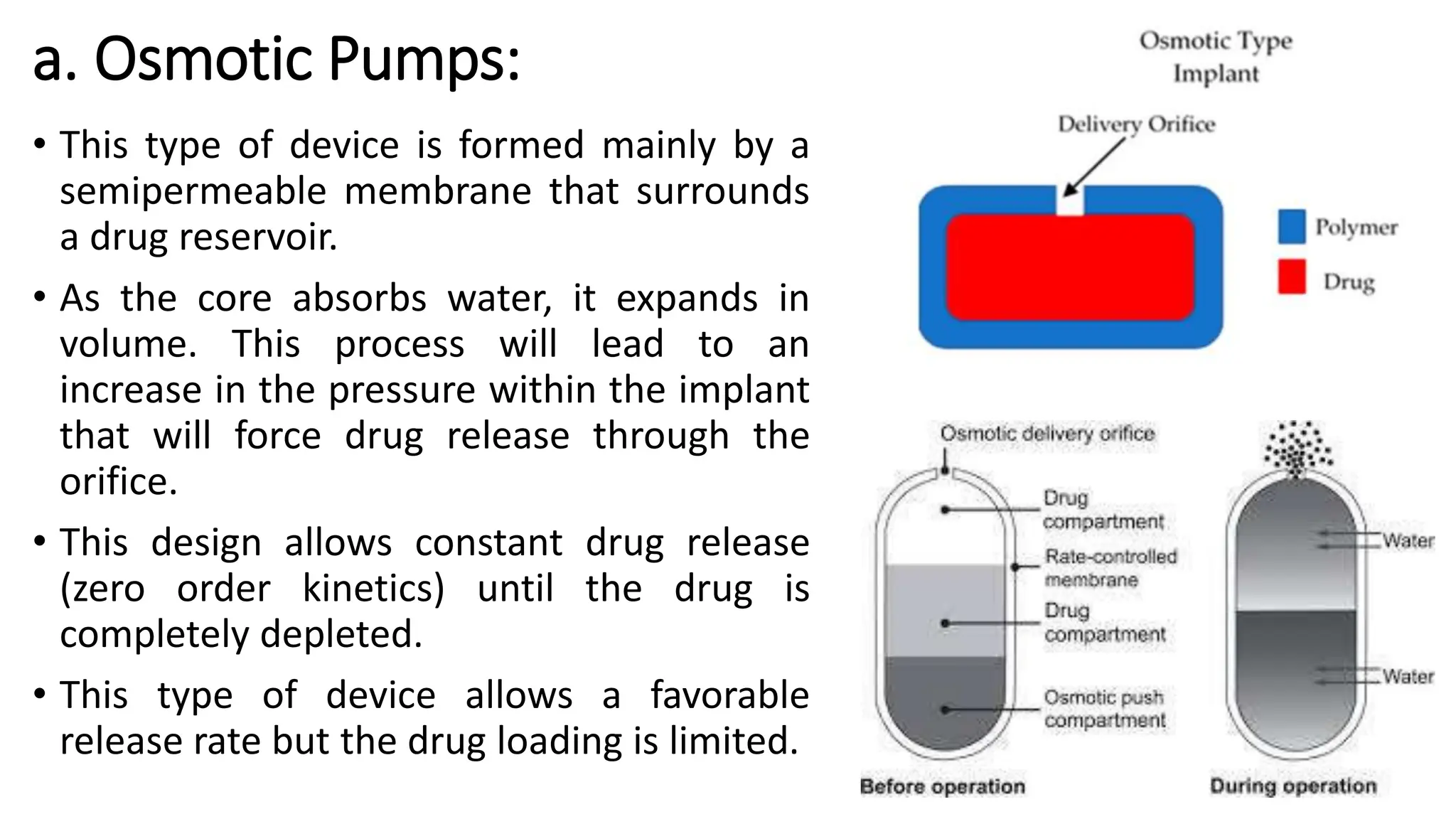
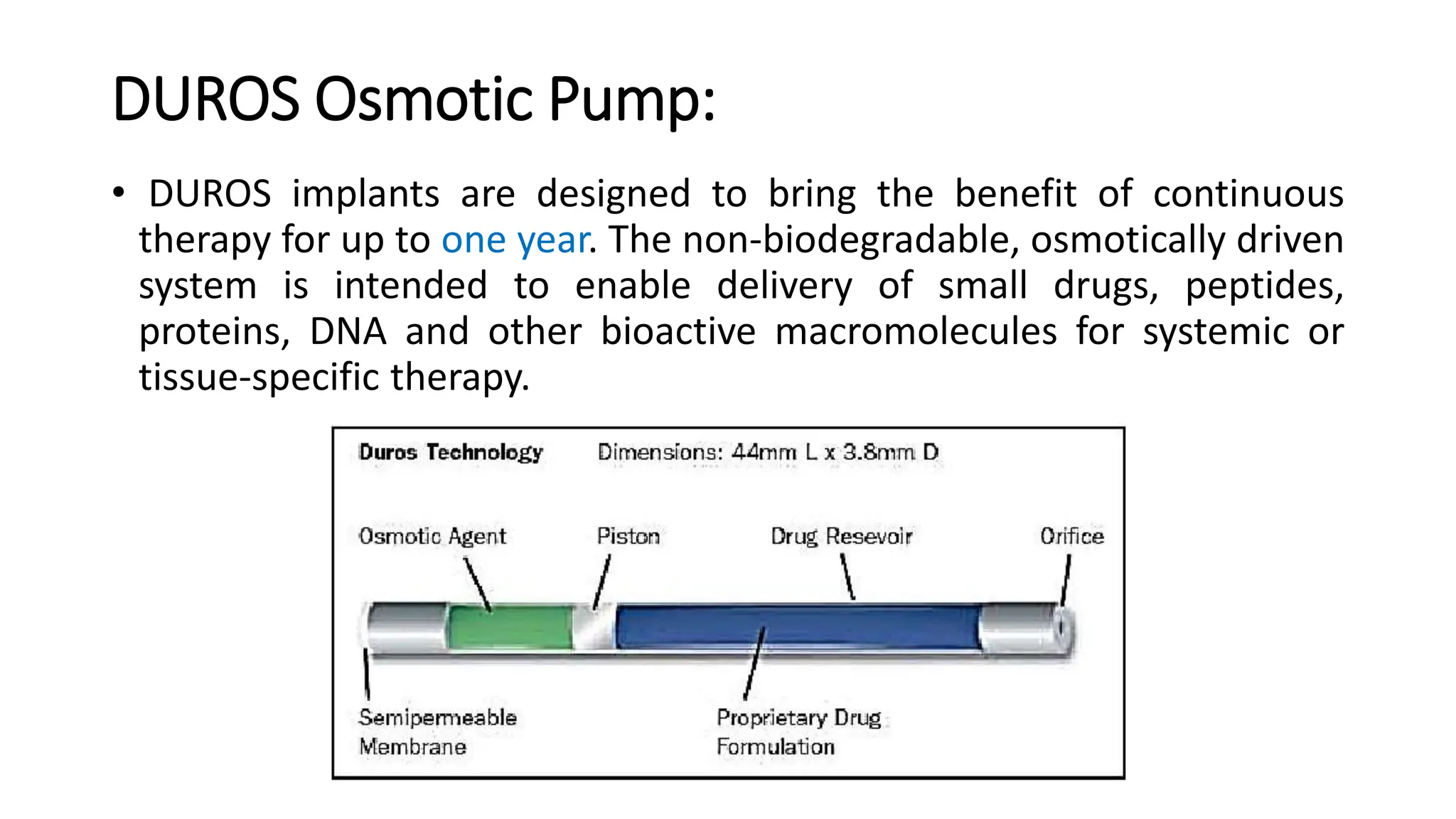
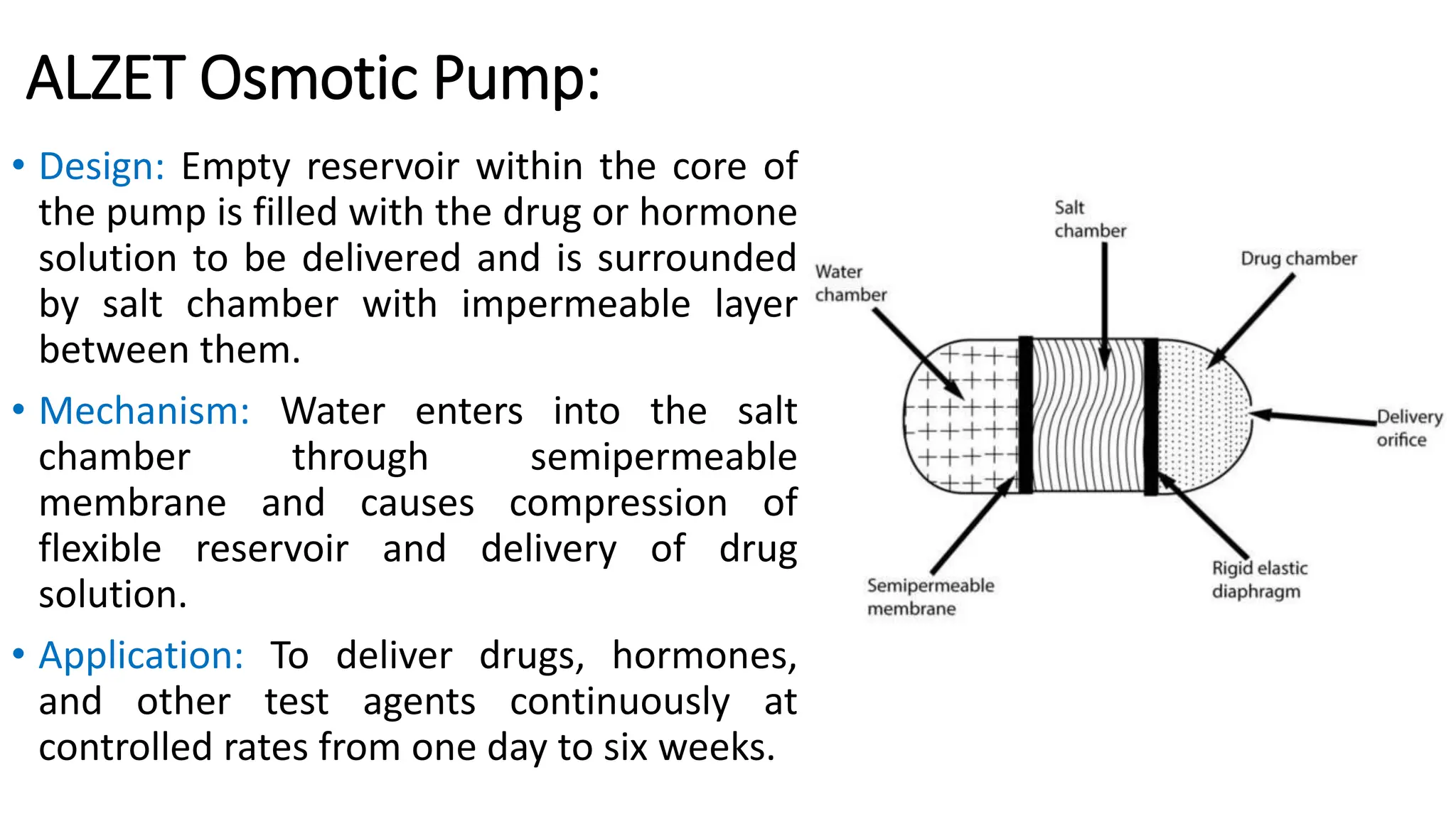
The document discusses implantable drug delivery systems that allow targeted and localized drug delivery to enhance patient compliance and minimize side effects. It includes details on types of implants, their advantages and disadvantages, and the mechanisms of drug release. Various applications in women's health, chronic diseases, and other medical fields are also outlined.