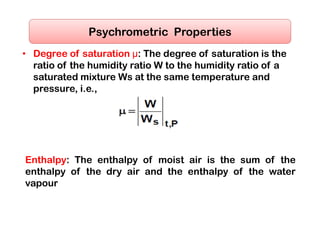
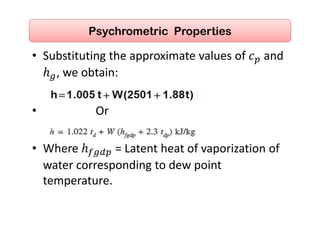
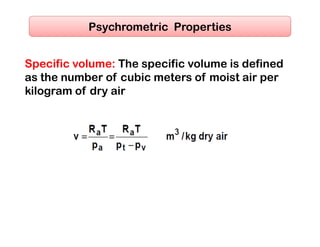
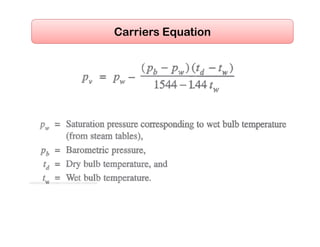
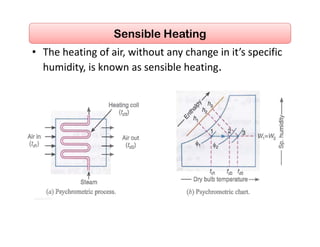
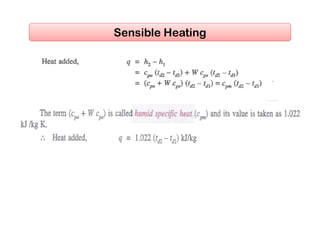
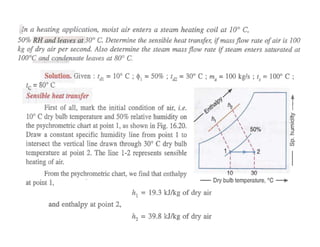
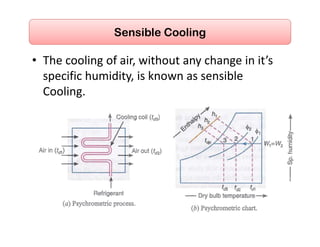
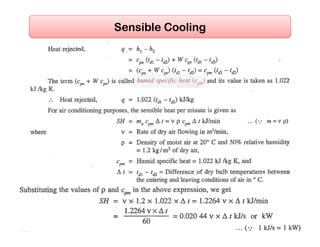
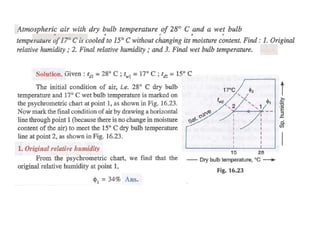
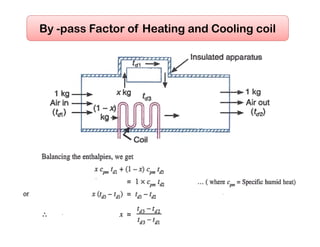
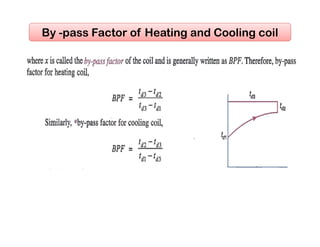
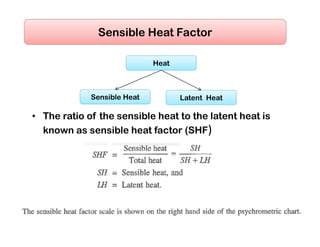
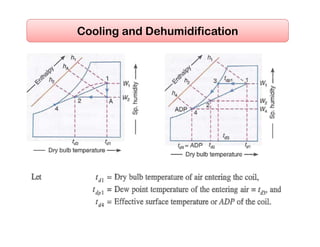
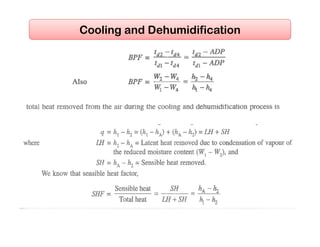
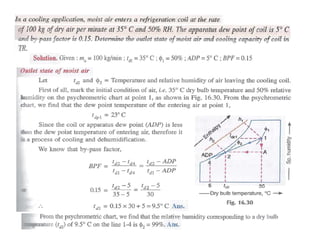
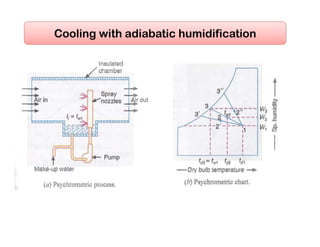
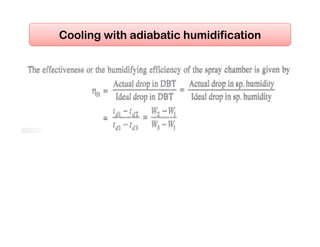
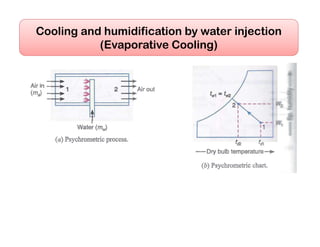
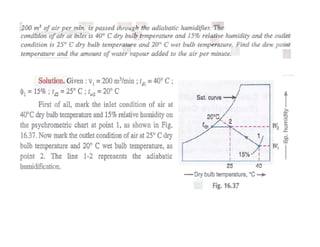
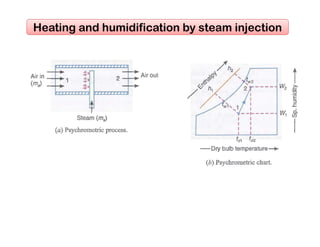
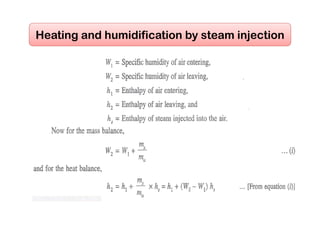
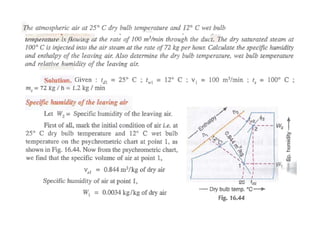
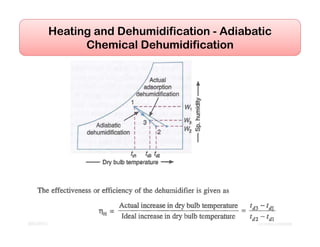
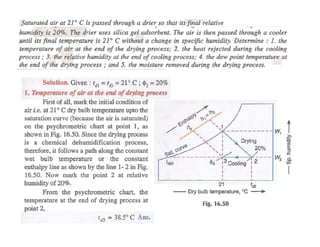
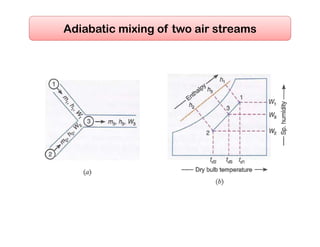
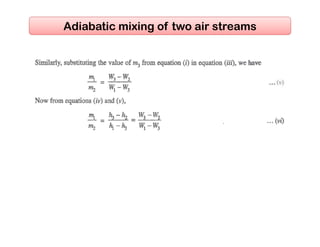
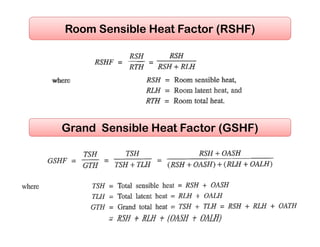
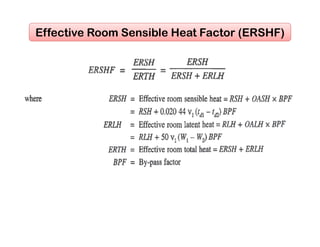
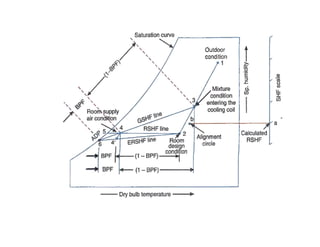
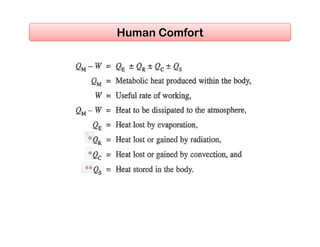
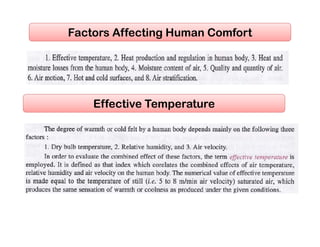
This document discusses psychrometrics and air conditioning load estimation. It covers topics like:
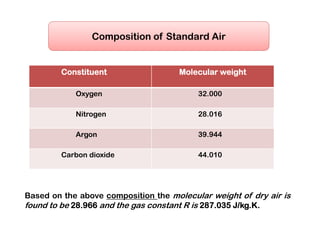
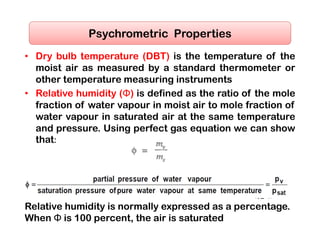
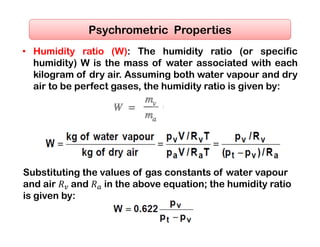
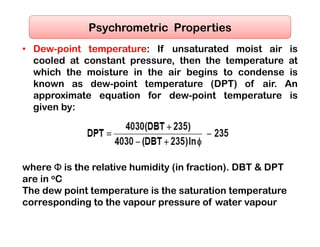
- The composition of air and properties of moist air like humidity ratio, enthalpy, specific volume.
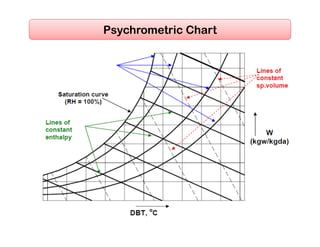
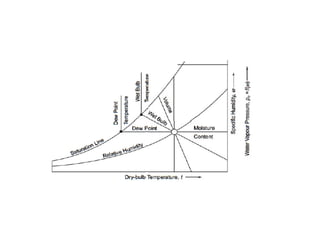
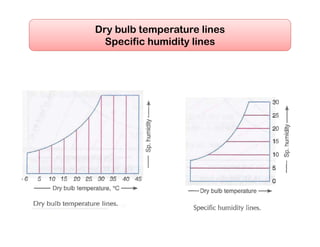
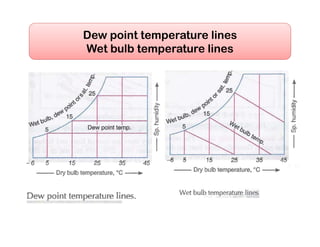
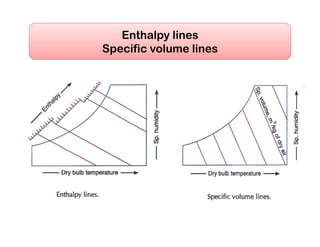
- Psychrometric chart which graphically represents thermodynamic properties of moist air.
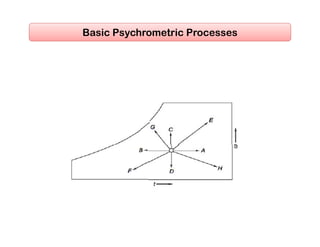
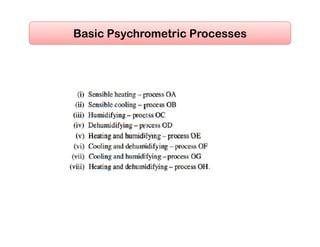
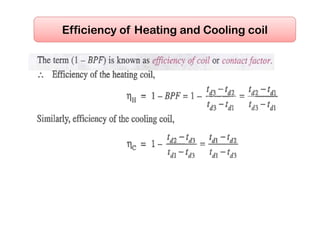
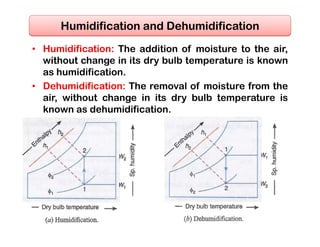
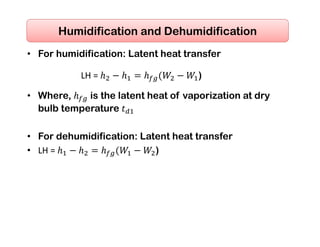
- Basic psychrometric processes like sensible heating/cooling, humidification, dehumidification.
- Methods to achieve these processes like air washers, evaporative cooling, steam injection.
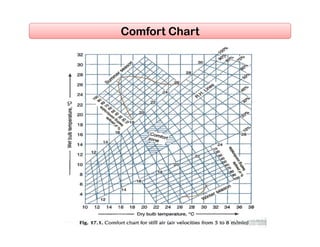
- Factors affecting human comfort and the use of comfort charts.
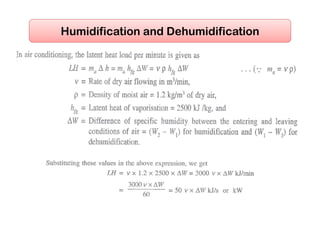
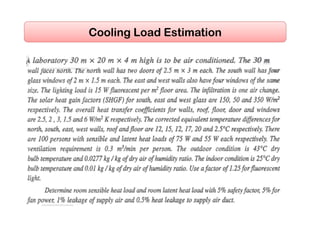
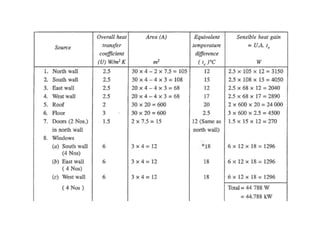
- Estimating cooling loads for air conditioning systems.