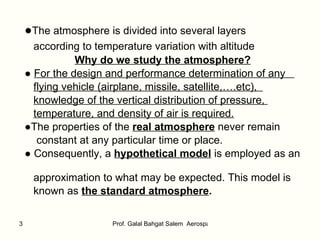
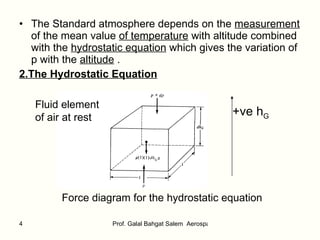
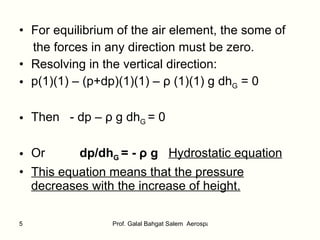
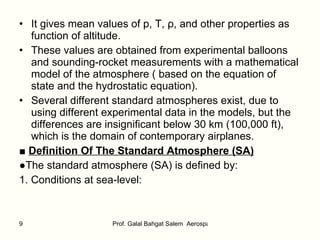
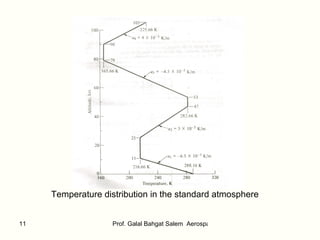
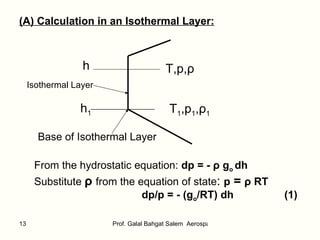
The document summarizes the structure and properties of the standard atmosphere model used in aerospace engineering. It describes the standard atmosphere as divided into layers of either constant temperature or linear temperature gradients. Equations are provided to calculate how pressure, temperature, and density vary with altitude based on the temperature profile and hydrostatic equation. Standard atmosphere tables in appendices provide mean property values as a function of altitude that serve as a reference for aircraft and vehicle design and performance calculations.


![What is the altitude? ● There are six (6) different altitudes: 1-Geometric Altitude h G is the height above sea-level. 2-Absolute Altitude h a is the height measured from the center of the earth. h a = h G + R where R is the radius of earth ( R = 6356.766 km) The absolute altitude is important for space flight because g varies with h a . ● According to Newton’s law of gravity, g = g o [R/h a ] 2 = g o [R/(R + h G ) ] 2 where g o is g at sea-level where 3-Geopotential Altitude h is a fictitious (hypothetical) altitude used to facilitate the calculations ( h ≠ h G )](https://image.slidesharecdn.com/thestandardatmosphere-110226120937-phpapp01/85/The-standard-atmosphere-6-320.jpg)
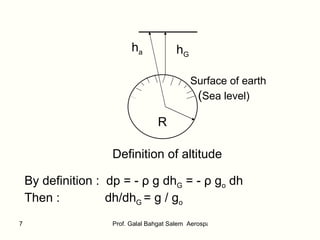
![What is the relation between h & h G ? dh = R 2 /(R+h G ) 2 dh G Integrate: 0 ∫ h dh = 0 ∫ h G R 2 /(R+h G ) 2 dh G The result is: h = [R/(R+h G )] h G At h G = 6.5 km , h is less than h G by about 0.1% only. At h G = 65 km , h is less than h G by about 1% only. The Standard Atmosphere (SA) It is defined in order to relate flight tests, wind-tunnel tests, and the general design and performance of flying vehicle ( aircraft, missile, satellite,….etc ) to a common reference.](https://image.slidesharecdn.com/thestandardatmosphere-110226120937-phpapp01/85/The-standard-atmosphere-8-320.jpg)


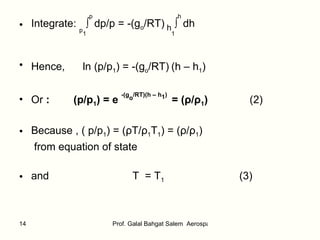
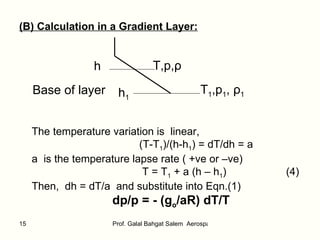
![Integrate: p1 ∫ p dp/p = -(g o /aR) T1 ∫ T dT/T Hence, ln p/p 1 = - (g o /aR) ln T/T 1 Then, p/p 1 = (T/T 1 ) -g o /aR (5) From equation of state: ( p/p 1 ) = ( ρ T/ ρ 1 T 1 ) = ( ρ / ρ 1 )(T/T1) Hence, ( ρ / ρ 1 ) = (T/T1) -[(g o /aR)+1] (6) Where T is calculated from Eq.(4)](https://image.slidesharecdn.com/thestandardatmosphere-110226120937-phpapp01/85/The-standard-atmosphere-16-320.jpg)
