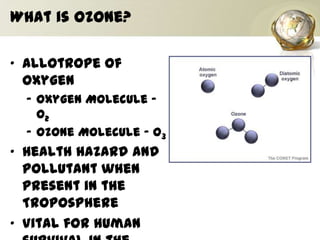
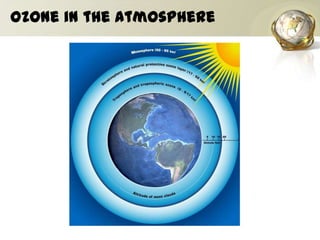
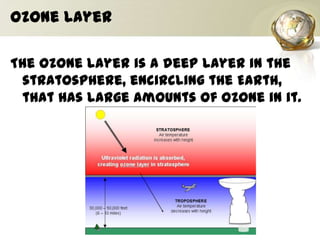
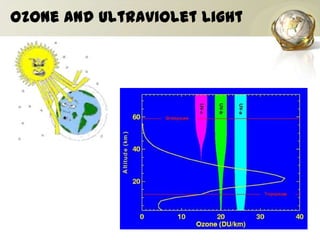
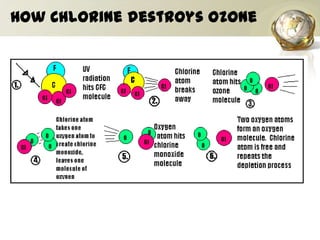
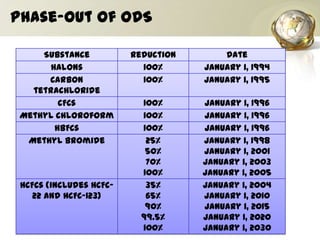
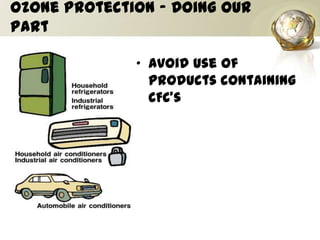
The document discusses ozone, its importance for protecting life on Earth from UV radiation, and the problem of ozone depletion caused by ozone depleting substances (ODS) like CFCs. It notes that ozone exists in different layers of the atmosphere and that the ozone layer in the stratosphere shields the planet from harmful UV rays. It then explains how certain man-made chemicals released into the air can destroy ozone molecules, leading to ozone depletion. The document outlines international agreements like the Montreal Protocol to phase out the production of ODS and protect the ozone layer. It concludes by suggesting individual actions people can take to help limit ozone depletion.