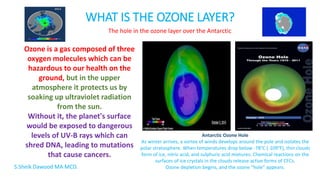
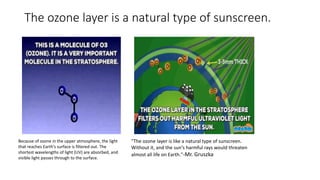
The ozone layer protects life on Earth by absorbing harmful ultraviolet radiation from the Sun. Ozone is produced in the stratosphere when oxygen molecules interact with solar radiation. Without the ozone layer, dangerous levels of UV radiation would reach the surface and threaten life. In the 1980s, scientists discovered that chlorofluorocarbons (CFCs) were depleting the ozone layer by triggering chemical reactions that break down ozone molecules. This caused the formation of the ozone hole over Antarctica. The 1987 Montreal Protocol banned CFCs and has led to a slow recovery of the ozone layer over the past 30 years.