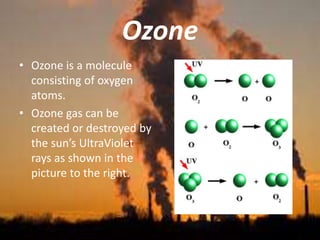
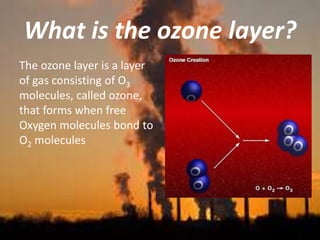
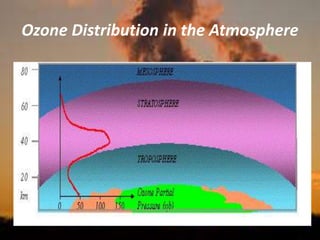
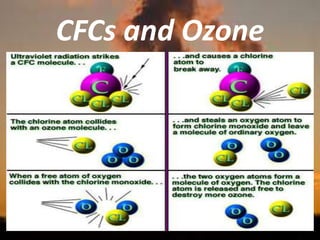
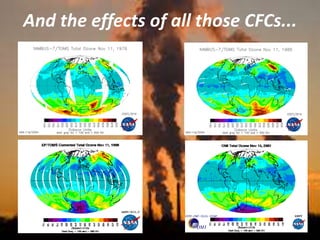
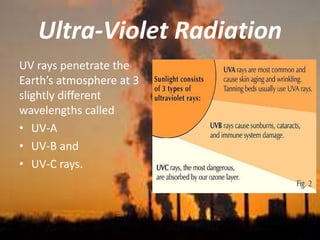
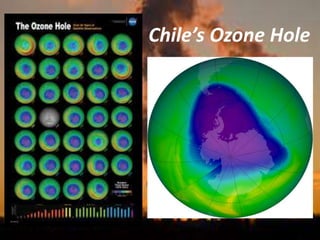
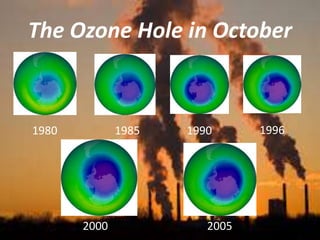
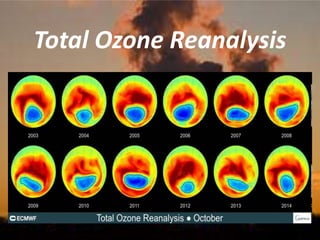
International Ozone Day is presented to discuss the ozone layer and the threats to it. The ozone layer protects life on Earth by absorbing ultraviolet radiation from the sun. Chlorofluorocarbons (CFCs) released into the atmosphere were depleting the ozone layer. This led to the discovery of the ozone hole over Antarctica in 1985. In response, the Montreal Protocol was established in 1987 to phase out the production of ozone-depleting substances and allow the ozone layer to recover by 2050 to 2075.