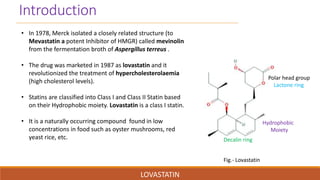
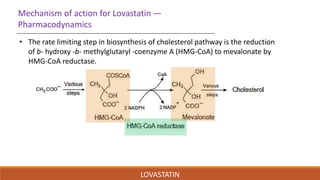
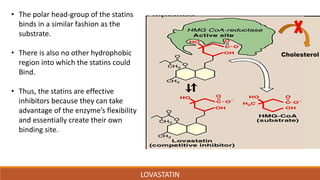
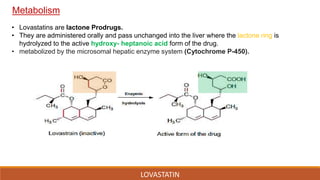
Lovastatin was the first statin drug approved for treatment of high cholesterol. It was isolated from fermentation of Aspergillus terreus in 1978 and marketed in 1987. Lovastatin is a class I statin with a polar head group and hydrophobic moiety. It works by competitively inhibiting HMG-CoA reductase, the rate limiting enzyme in cholesterol biosynthesis. Lovastatin is administered orally and converted to its active form in the liver. It has a biological half-life of 13.37 hours and is metabolized and eliminated primarily in the feces. Adverse effects include myopathy and rhabdomyolysis.