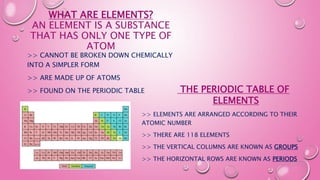
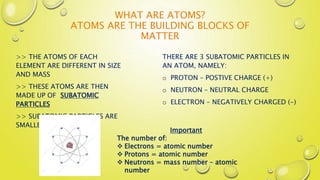
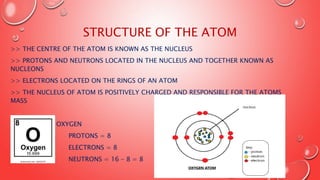
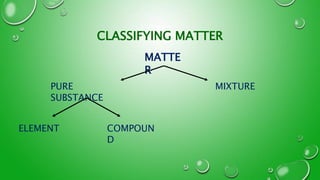
This document provides an introduction to basic chemistry concepts including matter, elements, atoms, and the structure of the atom. It defines matter as anything that has mass and takes up space. Elements are made of only one type of atom and can be found on the periodic table, which arranges elements by their atomic number and properties. Atoms are the building blocks of matter and are made up of protons, neutrons, and electrons. The nucleus of an atom, containing protons and neutrons, is at the center and electrons orbit around it. The document also distinguishes between pure substances like elements and compounds, and mixtures which can be separated physically.