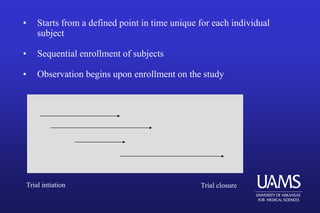
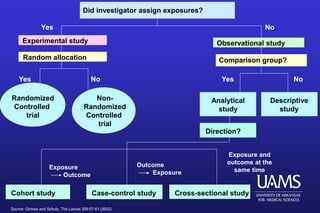
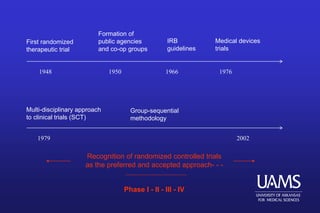
This document provides an overview of clinical trials and the role of statistics within clinical research. It discusses how clinical trials allow researchers to systematically study medical treatments and make evidence-based inferences about a treatment's effects. The document outlines the key elements of clinical trials, such as using a control group, randomization, blinding, and informed consent. It also discusses the history of clinical trials and how they became the preferred method for medical research due to providing a standardized and ethical way to study human subjects compared to observational studies. Finally, the document addresses some of the ethical considerations around clinical trials, such as balancing patient welfare with gaining scientific knowledge.