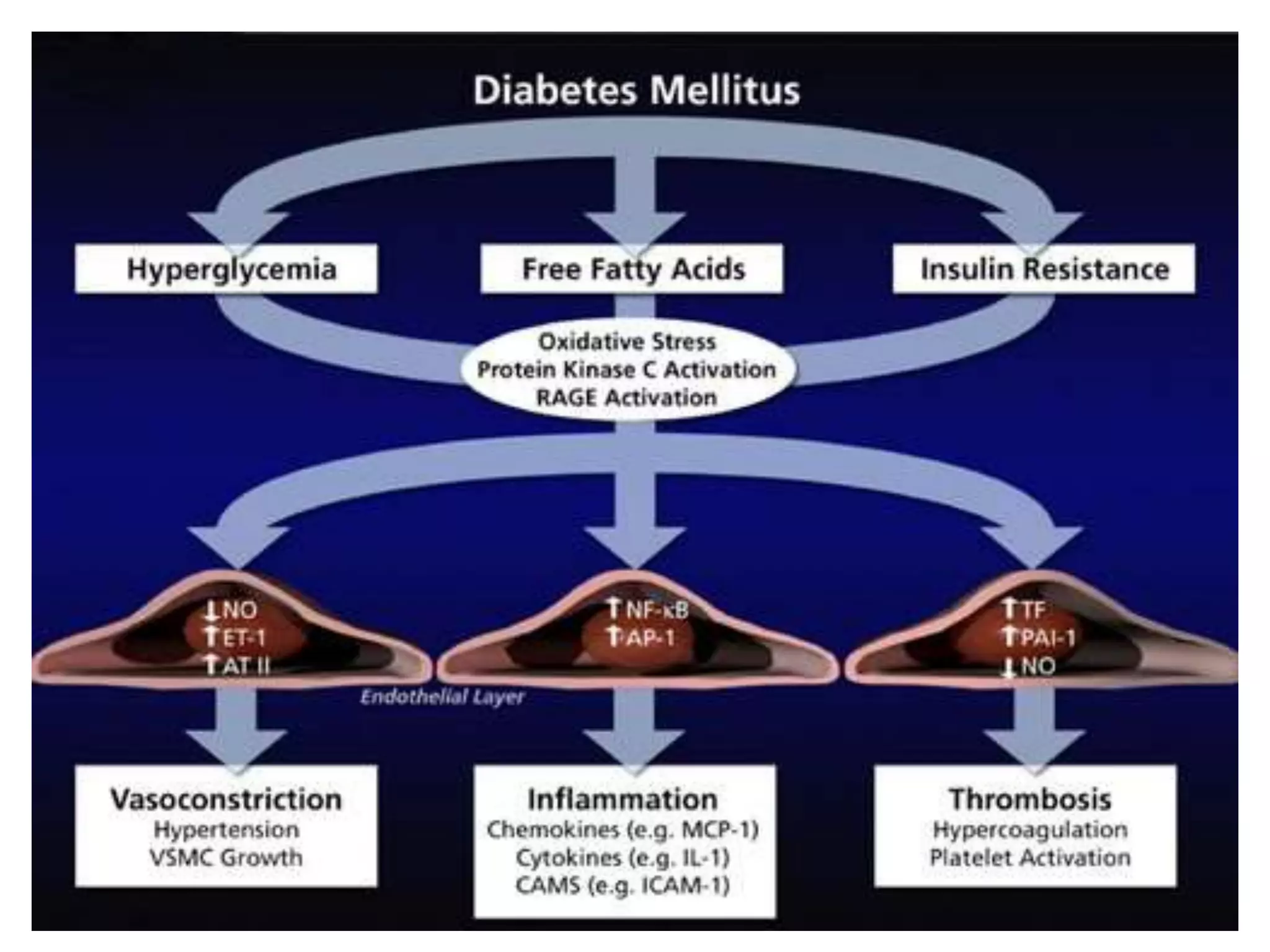
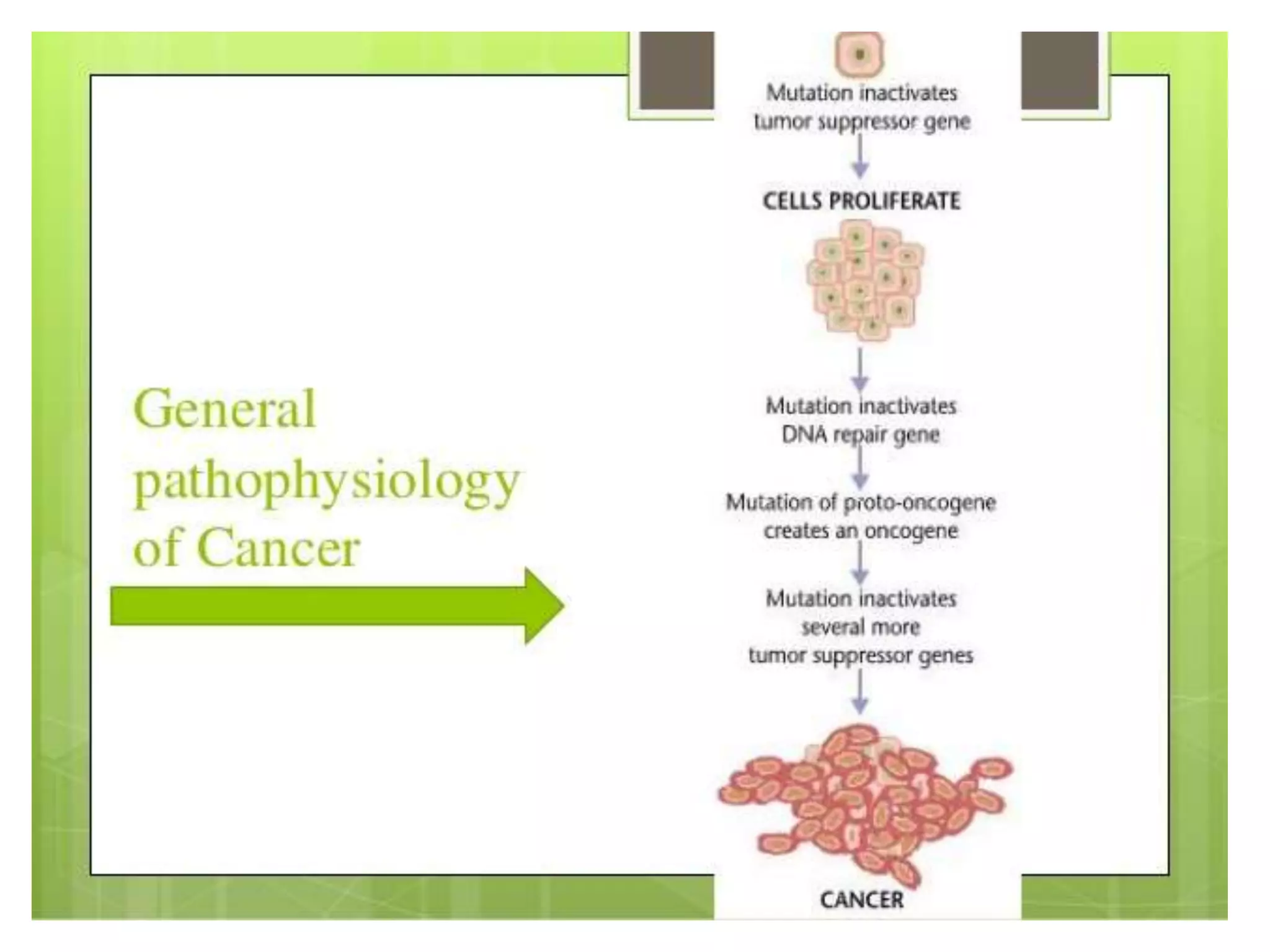
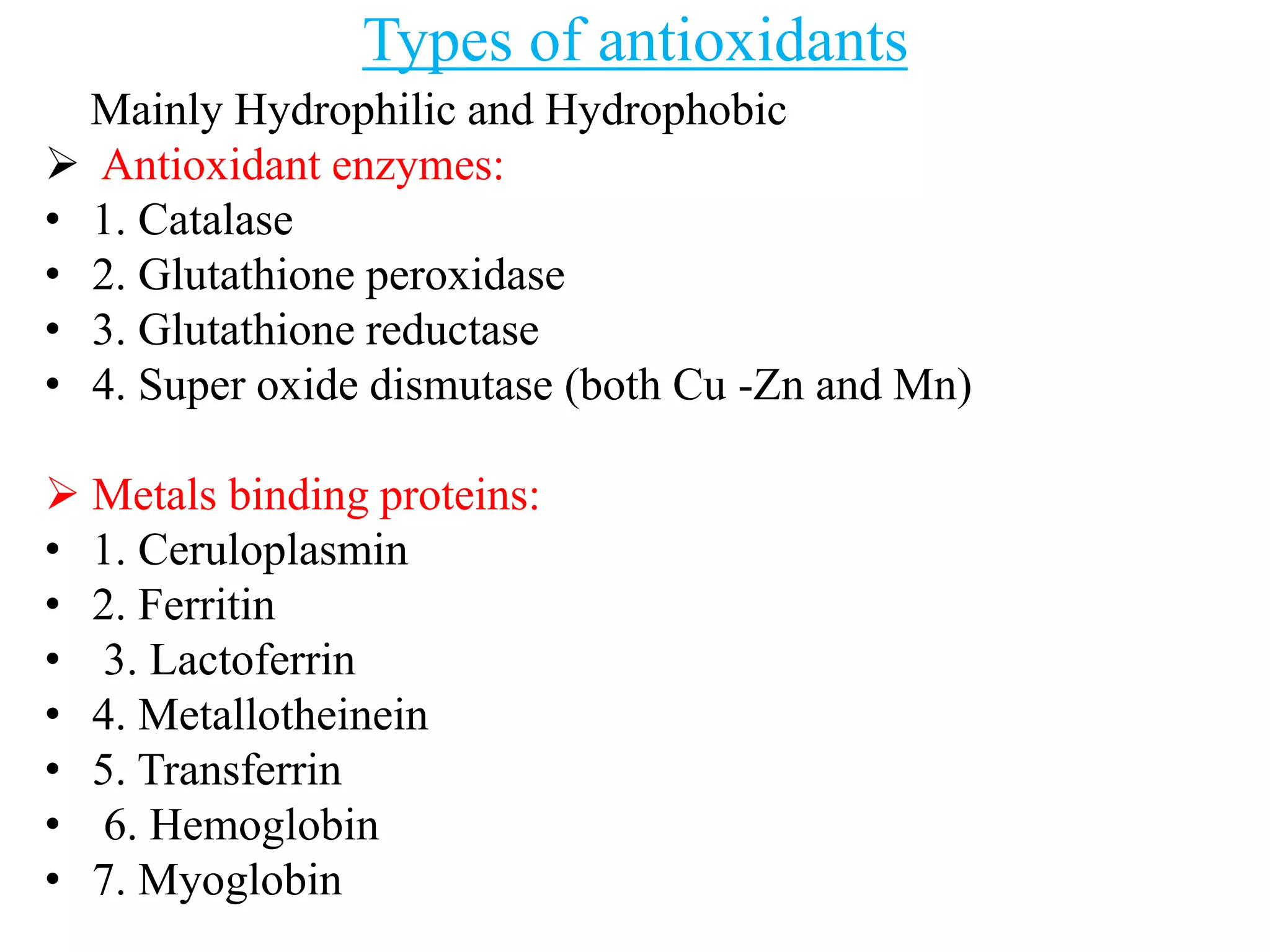
This document summarizes the pharmacology of free radicals and their role in various diseases. It discusses how free radicals are generated and the types of free radicals. It then describes how free radicals cause oxidative damage and lead to disorders like diabetes, neurological diseases, cancer, and rheumatoid arthritis. The document also outlines various antioxidants that can protect against free radical damage.