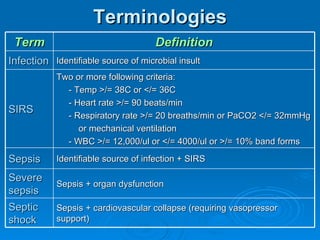
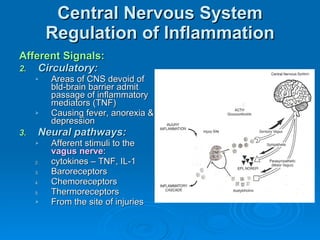
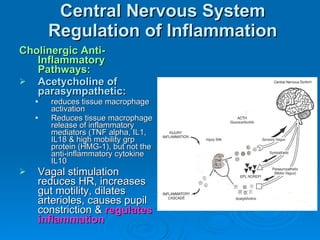
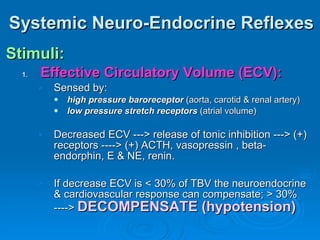
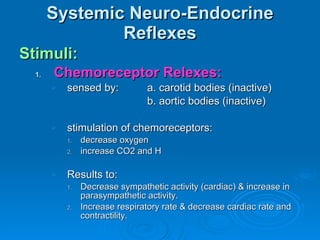
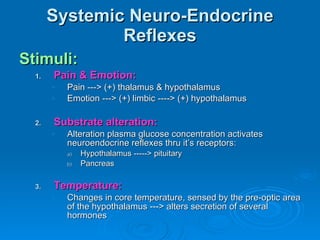
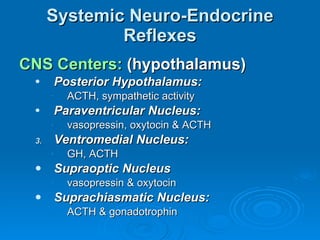
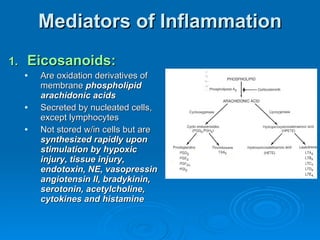
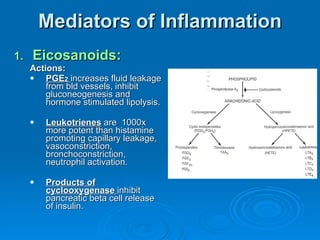
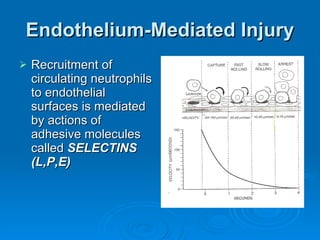
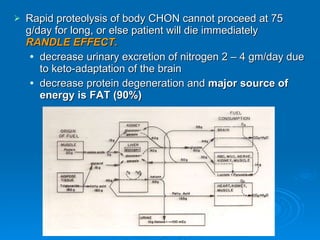
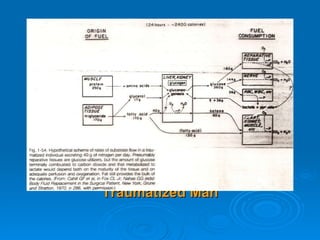
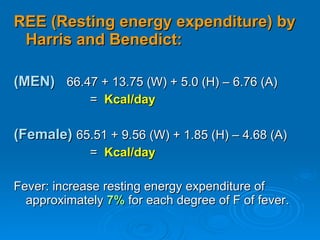
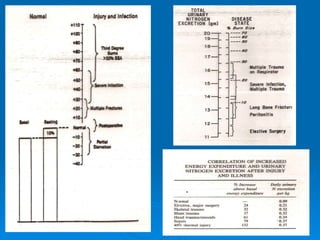
This document summarizes the systemic response to injury and infection in the body. It discusses how injuries trigger neuroendocrine and inflammatory responses to restore homeostasis. The response has two phases - an initial pro-inflammatory response to fight infection, followed by an anti-inflammatory phase to prevent excessive inflammation and restore homeostasis. It provides details on the hormones, cytokines and other mediators involved in this response, including their effects on various organ systems.