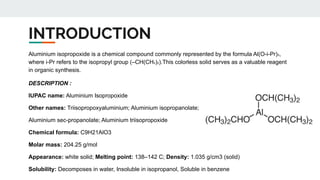
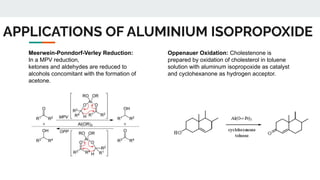
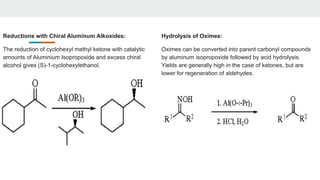
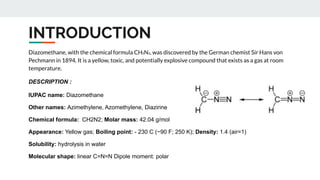
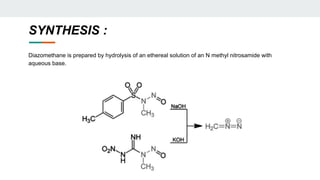
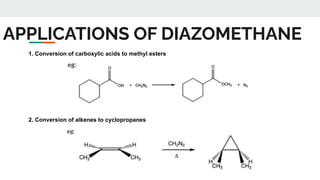
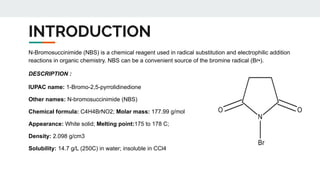
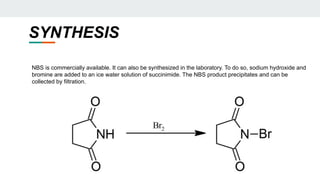
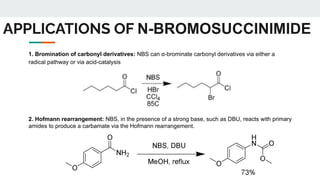
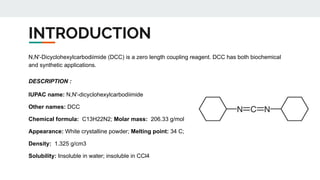
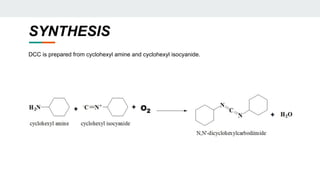
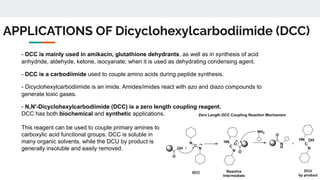
The document provides a detailed overview of various synthetic reagents including aluminium isopropoxide, diazomethane, n-bromosuccinimide, dicyclohexylcarbodiimide, wittig reagent, and Wilkinson’s reagent, outlining their chemical properties, synthesis methods, and applications in organic synthesis. Each reagent is described with its IUPAC name, formula, appearance, and specific uses, such as catalysis and reductions. Overall, it emphasizes the importance of these reagents in facilitating various chemical transformations in pharmaceutical chemistry.



![SYNTHESIS
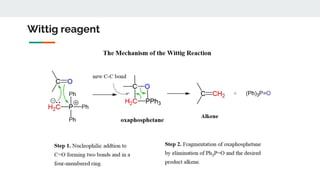
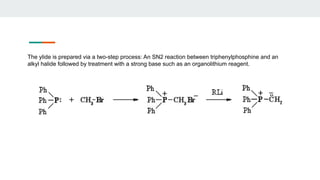
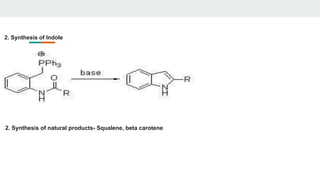
1. Preparation of Phosphorus Ylides - Wittig reagents are usually prepared from a phosphonium salt, which is in
turn prepared by the quaternization of triphenylphosphine with an alkyl halide.
The alkyl phosphonium salt is deprotonated with a strong base such as n-butyllithium:
[Ph3P+CH2R]X− + C4H9Li → Ph3P=CHR + LiX + C4H1
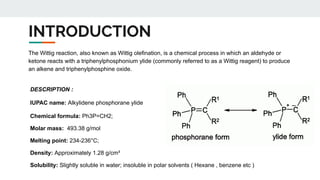
2. Structure of the Ylide - The Wittig reagent may be described in the phosphorane form (the more familiar
representation) or the ylide form.
- The ylide form is a significant contributor, and the carbon atom is nucleophilic.](https://image.slidesharecdn.com/syntheticreagentsandapplications-241120181603-8535b2b9/85/SYNTHETIC-REAGENTS-AND-APPLICATIONS-PRESENTATION-22-320.jpg)
![INTRODUCTION
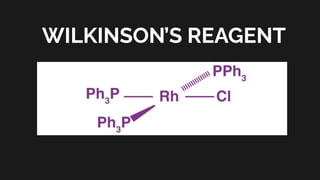
Wilkinson's reagent is a well-known organometallic compound, primarily used as a catalyst for the
hydrogenation of alkenes. It is a red solid and a coordination complex of rhodium with
triphenylphosphine ligands. Its efficient catalysis in homogeneous systems makes it valuable in
synthetic chemistry.
DESCRIPTION :
IUPAC name: Chloridotris(triphenylphosphine)rhodium(I)
Chemical formula: [RhCl(PPh₃)₃]
Molecular mass: 925.22 g/mol
Density: Approximately 1.55 g/cm³
Solubility: Soluble in organic solvents like benzene, toluene, and dichloromethane; insoluble in water](https://image.slidesharecdn.com/syntheticreagentsandapplications-241120181603-8535b2b9/85/SYNTHETIC-REAGENTS-AND-APPLICATIONS-PRESENTATION-27-320.jpg)
![SYNTHESIS
Wilkinson's reagent is synthesized by reacting
rhodium(III) chloride hydrate(RhCl₃·3H₂O) with
triphenylphosphine (PPh₃) in ethanol or similar solvents.
The reaction involves reducing rhodium from the
+3 oxidation state to the +1 state. After stirring the mixture,
the product, [RhCl(PPh₃)₃], is isolated as a red crystalline solid.](https://image.slidesharecdn.com/syntheticreagentsandapplications-241120181603-8535b2b9/85/SYNTHETIC-REAGENTS-AND-APPLICATIONS-PRESENTATION-28-320.jpg)


