This document describes research into the development of a catalytic method for synthesizing α-carbonyl imines through the reaction of α-diazocarbonyl compounds and organic azides under mild conditions. Dirhodium tetraacetate catalyzes this reaction to produce imines containing polar functional groups like ketones and α-diesters in overnight reactions. The document also discusses the synthesis of 1,2,3,4-tetrahydropyrimidines from a [4+2] cycloaddition of an azadiene and aldimines catalyzed by zinc triflate. Finally, it presents a rhodium-catalyzed Wolff rearrangement and [2+2] cycloaddition that yields novel β-lact
![1
ABSTRACT
Title of Document: A CATALYTIC METHOD FOR THE
SYNTHESIS OF α-CARBONYLIMINES: NOVEL
ROUTES TO BIOLOGICALLY ACTIVE
MOLECULES
Michael D. Mandler, Bachelor of Science, 2015
Directed By: Professor Michael P. Doyle, Department of
Chemistry and Biochemistry
A dirhodium tetraacetate-catalyzed reaction between α-diazocarbonyl compounds and
organic azides produces imines after overnight reaction times and mild conditions. Imines
containing polar functional groups, such as ketones and α-diesters can be easily
synthesized via this method. Moreover, an unprecedented zinc triflate-catalyzed [4+2]
cycloaddition between an azadiene and aldimines yielded functionalized 1,2,3,4-
tetrahydropyrimidines in good yields. A rhodium-catalyzed Wolff rearrangement of a
phenyldiazoacetoacetate enone followed by a [2+2] cycloaddition yielded novel β-
lactams in high yields and high diastereoselectivities. This methodology, extended to a
one-pot, multicomponent reaction, was developed to convert azide and diazo precursors
directly to β-lactams in almost quantitative yields.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-1-320.jpg)



![5
TABLE OF CONTENTS
List of Abbreviations ........................................................................................................ 6
Chapter 1: Catalytic Reaction Between Azide and Diazo Compounds: A Portal to
Novel Imines ...................................................................................................................... 7
1.1
Introduction: The Metal-Catalyzed Imine Forming Reaction........................................7
1.2 Overview of Metal Carbenes Derived from Diazo Compounds........................................9
1.3 Azide Trapping of Carbenes ............................................................................................10
1.4 Synthesis of Diazo Compounds.......................................................................................12
1.5 Synthesis of Alkyl and Aryl Azides.................................................................................14
1.6 Reaction and Optimization ..............................................................................................15
1.7 Hydrolysis of Methyl 2-(Benzylimino)-3-oxobutanoate .................................................16
1.8 Aryl Azides Yield More Stable N-Aryl Imines ...............................................................18
1.9 Optimization ....................................................................................................................19
1.10 Donor/Acceptor Carbene Precursors Improve Yield.....................................................20
1.11 The Stereochemistry of Imine Products.........................................................................22
1.12 Substrate Scope..............................................................................................................22
1.13 Experimental..................................................................................................................23
Chapter 2: Synthesis of 1,2,3,4-Tetrahydropyrimidines from Novel [4+2]
Cycloadditions Between an Azadiene and Aldimines.................................................. 33
2.1 Background......................................................................................................................33
2.2 Substrate Scope................................................................................................................34
2.3 Experimental....................................................................................................................35
Chapter 3: Synthesis of β-Lactams by [2+2] Cycloadditions between Ketene and
Imines............................................................................................................................... 43
3.1 Background......................................................................................................................43
3.2 Metal-Catalyzed Wolff Rearrangement...........................................................................43
3.3 Staudinger Synthesis........................................................................................................44
3.4 Structural Assignment of β-Lactams 23 ..........................................................................45
3.5 Substrate Scope................................................................................................................46
3.6 One-Pot Synthesis of β-Lactams......................................................................................47
3.7 Experimental....................................................................................................................49
Chapter 4: NMR Spectra ............................................................................................... 67
References........................................................................................................................ 93](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-5-320.jpg)

![8
at the nitrogen atom, allowing for reactions such as the Staudinger synthesis ([2+2]
cycloaddition between ketenes and imines) which has been a major route for the
preparation of β-lactams for over a century.7,8
Imines are versatile intermediates for the
synthesis of biologically relevant molecules, particularly alkaloids. Hence, the installation
of imine functional groups, especially in molecules containing complex functionalities, is
a topic of high importance to chemists.
The conventional synthesis of imines involves the condensation of a primary
amine with a carbonyl compound such as an aldehyde or ketone (Scheme 1). The water
formed is often removed by distillation using a Dean-Stark apparatus or by running the
reaction in a solvent containing anhydrous MgSO4 or another suitable drying agent.
Scheme 1. Conventional condensation route to imines
While the condensation methodology is the method of choice for making simple imines,
it is not compatible for producing complex imines; for example, those containing
condensable carbonyl groups such as 1 (Scheme 2). Though the central carbonyl group in
the vicinal tricarbonyl system 2 is the most susceptible to attack by the primary amine 3,
the condensation reaction would be expected to yield multiple products. Hence, another
chemical route is necessary for the efficient synthesis of 1.
Scheme 2. Imine formation complicated by multiple carbonyl groups](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-8-320.jpg)

![11
intramolecular reaction between an azide functional group and rhodium(II) carbene
generated from 13 (Scheme 5).30
After screening various catalysts, they observed that the
less-electrophilic Rh2(cap)4 and Rh2(acam)4 provided 14 in higher yield (determined by
1
H NMR spectroscopy).
Scheme 5. Lecourt and Micouin’s intramolecular reaction30
The authors proposed a mechanism for this intramolecular transformation (Scheme 6).
First, a dirhodium metal carbene [13a] is generated, which then is attacked by the internal
nitrogen of the azide to form a six-membered ring [13b]. Lastly, a second molecule of
dinitrogen is lost from the azide group to form the endocyclic imine 14.
Scheme 6. Lecourt and Micouin’s proposed mechanism](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-11-320.jpg)

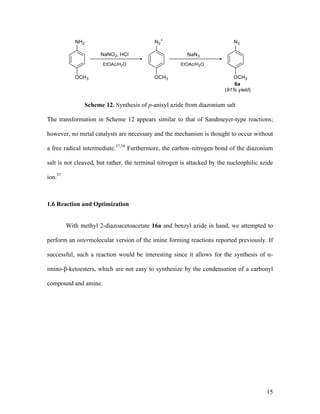
![13
Dichloromethane (DCM) was used instead of acetonitrile because DCM is easier
to remove during the purification process. However, when the diazo transfer reaction is
performed in DCM instead of MeCN, the reaction rate is slower. Thus, overnight reaction
times were needed. Diazoacetoacetates such as methyl 2-diazoacetoacetate 16a (R1
= Ac)
and dimethyl diazomalonate 16b were synthesized using triethylamine as the base.
Methyl phenyldiazoacetate 16c (R1
= Ph) was made in a similar fashion, except that the
phenylacetic acid ester was treated with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), a
stronger base than triethylamine, in order to deprotonate the less acidic α-methylene
protons (Scheme 9).
Scheme 9. Diazo transfer reactions of common α-diazocarbonyl compounds1
Silyl enol diazoacetate 16d was synthesized following a known procedure33
by
treating 16a with tert-butyldimethylsilyl trifluoromethanesulfonate (TBSOTf) and Et3N
(Scheme 10). The reaction was monitored by 1
H NMR spectroscopy, and then 16d was
purified by aqueous work-up (16d is not silica stable).](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-13-320.jpg)

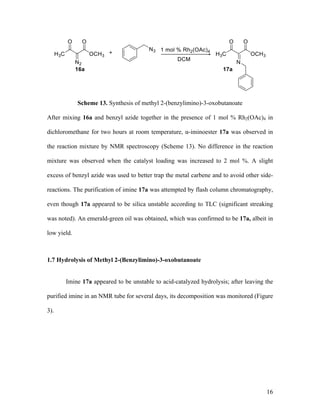




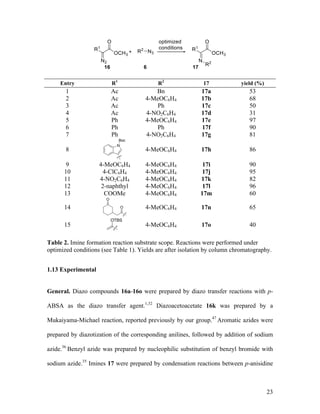

![25
via a syringe pump over the course of 1 h. The reaction mixture was allowed to stir for 24
h at reflux. After cooling, the solvent was removed in vacuo, and the residue was purified
by silica gel column chromatography to provide imines 17. The major imine isomer was
assigned the Z configuration by analogy to a known compound, 17g, whose
stereochemistry was verified by X-ray crystallography in the literature.44
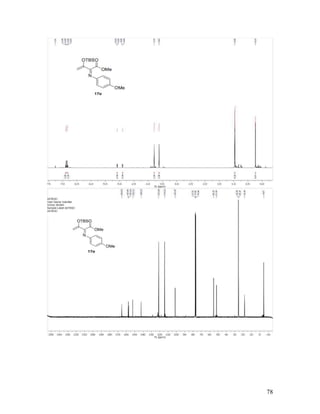
Methyl 2-(Benzylimino)-3-oxobutanoate (17a). Reaction between
diazoacetoacetate 16a and benzyl azide followed by flash chromatographic purification
gave 17a as a single geometric isomer in 53% yield; 17a is not stable on TLC and was
found to decompose on the silica gel column, leaving behind a green streak. Green liquid;
TLC Rf = 0.55 (4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.41 – 7.28 (comp,
5H), 4.74 (s, 2H), 3.92 (s, 3H), 2.46 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 197.0, 164.0,
159.5, 137.0, 128.7, 128.0, 127.6, 59.1, 52.4, 24.6; IR (neat) 1715, 1702 cm-1
; HRMS
(ESI) m/z calculated for C12H14NO3 [M+H]+
220.0974, found: 220.0981.
Methyl 2-(4-Methoxyphenyl)imino-3-oxobutanoate (17b). Reaction between
diazoacetoacetate 16a and p-anisyl azide 6a followed by chromatographic purification
gave 17b as a single geometric isomer in 68% isolated yield. Red liquid; TLC Rf = 0.35](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-25-320.jpg)
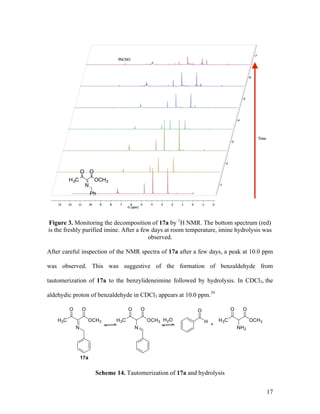
![26
(4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 9.0 Hz, 2H), 6.90 (d, J
= 9.0 Hz, 2H), 3.83 (s, 3H), 3.77 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 197.1, 165.3,
159.7, 154.6, 139.6, 123.3, 114.5, 55.5, 52.4, 24.6; IR (neat) 1737, 1695 cm-1
; HRMS
(ESI) m/z calculated for C12H14NO4 [M+H]+
236.0923, found: 236.0941.
Methyl 3-Oxo-2-(phenylimino)butanoate (17c). Reaction between
diazoacetoacetate 16a and phenyl azide 6b followed by chromatographic purification
gave 17c as a single geometric isomer in 50% isolated yield. Yellow liquid; TLC Rf = 0.5
(4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.41 – 7.35 (comp, 2H), 7.28 – 7.22
(m, 1H), 7.05 – 7.00 (comp, 2H), 3.69 (s, 3H), 2.57 (s, 3H); 13
C NMR (100 MHz, CDCl3)
δ 196.8, 164.1, 157.1, 147.2, 129.2, 127.3, 119.9, 52.3, 24.7; IR (neat) 1739, 1701 cm-1
;
HRMS (ESI) m/z calculated for C11H12NO3 [M+H]+
206.0817, found: 206.0819.
Methyl 2-(4-Nitrophenyl)imino-3-oxobutanoate (17d). Reaction between
diazoacetoacetate 16a and p-nitrophenyl azide 6c followed by chromatographic
purification gave 17d as one isomer in 31% yield. Orange solid; TLC Rf = 0.4 (4:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 8.26 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 9.0
Hz, 2H), 3.69 (s, 3H), 2.58 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 195.7, 162.5, 158.6,](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-26-320.jpg)
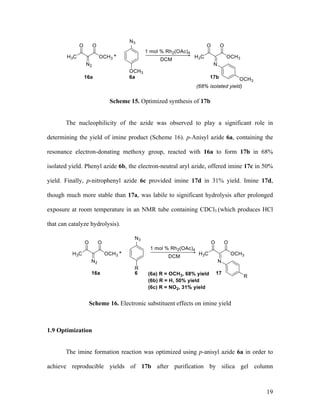
![27
152.7, 146.0, 124.9, 119.6, 52.7, 24.7; IR (neat) 1733, 1702 cm-1
; HRMS (ESI) m/z
calculated for C11H11N2O5 [M+H]+
251.0668, found: 251.0668.
Methyl 2-(4-Methoxyphenyl)imino-2-phenylacetate (17e). Reaction between
phenyldiazoacetate 16c and p-anisyl azide 6a followed by chromatographic purification
gave 17e as a mixture of geometric isomers (92:8 as measured by 1
H NMR spectrum) in
97% overall yield. 1
H and 13
C NMR spectral data is in accordance with the literature;48
however, we report a different coupling constant for the protons in the p-methoxyphenyl
group. Recrystallization in dichloromethane/hexanes gave yellow needles, mp = 89-91
°C; TLC Rf = 0.5 (4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) major isomer δ
7.89 – 7.81 (comp, 2H), 7.56 – 7.42 (comp, 3H), 6.97 (d, J = 9.0 Hz, 2H), 6.88 (d, J = 9.0
Hz, 2H), 3.81 (s, 3H), 3.70 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 166.1, 159.1, 157.3,
143.1, 134.1, 131.5, 128.7, 127.8, 121.2, 114.2, 55.4, 52.0; IR (neat) 1735, 1617 cm-1
;
HRMS (ESI) m/z calculated for C16H16NO3 [M+H]+
270.1130, found: 270.1133.
Methyl 2-Phenyl-2-(phenylimino)acetate (17f). Reaction between
phenyldiazoacetate 16c and phenyl azide 6b followed by chromatographic purification
gave 17f as a single geometric isomer in 90% isolated yield. Pale yellow solid, mp = 42-](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-27-320.jpg)
![28
44 °C; TLC Rf = 0.3 (6:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.92 – 7.84
(comp, 2H), 7.57 – 7.44 (comp, 3H), 7.38 – 7.31 (comp, 2H), 7.20 – 7.11 (m, 1H), 7.00 –
6.94 (comp, 2H), 3.64 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 165.4, 159.9, 150.0, 133.8,
131.8, 128.9, 128.7, 128.0, 125.0, 119.5, 51.9. IR (neat) 1730, 1622 cm-1
; HRMS (ESI)
m/z calculated for C15H14NO2 [M+H]+
240.1025, found: 240.1033.
Methyl 2-(4-Nitrophenyl)imino-2-phenylacetate (17g). Reaction between
phenyldiazoacetate 16c and p-nitrophenyl azide 6c followed by chromatographic
purification gave 17g as a single geometric isomer in 81% isolated yield. Pale yellow
solid, mp = 85-88 °C; TLC Rf = 0.4 (4:1 hexanes/EtOAc); 1
H and 13
C NMR spectral data
is in accordance with the literature;44
HRMS (ESI) m/z calculated for C15H13N2O4
[M+H]+
285.0875, found: 285.0865.
tert-Butyl 3-(2-Methoxy-1-(4-methoxyphenyl)imino-2-oxoethyl)-1H-indole-1-
carboxylate (17h). Reaction between 3-(N-Boc-indole)diazoacetate 16e and p-anisyl
azide 6a followed by chromatographic purification gave 17h as a single geometric isomer
in 86% isolated yield. Yellow solid, mp = 98-100 °C; TLC Rf = 0.4 (4:1](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-28-320.jpg)
![29
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 8.59 – 8.51 (m, 1H), 8.20 – 8.13 (m,
1H), 7.96 (s, 1H), 7.43 – 7.32 (comp, 2H), 6.99 (d, J = 9.0 Hz, 2H), 6.90 (d, J = 9.0 Hz,
2H), 3.82 (s, 3H), 3.68 (s, 3H), 1.70 (s, 9H). 13
C NMR (100 MHz, CDCl3) δ 165.4, 157.2,
154.1, 149.2, 143.6, 135.9, 130.5, 127.4, 125.6, 124.0, 123.2, 121.2, 116.7, 115.0, 114.2,
85.0, 55.4, 52.0, 28.1; IR (neat) 1742, 1732, 1607 cm-1
; HRMS (ESI) m/z calculated for
C23H25N2O5 [M+H]+
409.1763, found: 409.1758.
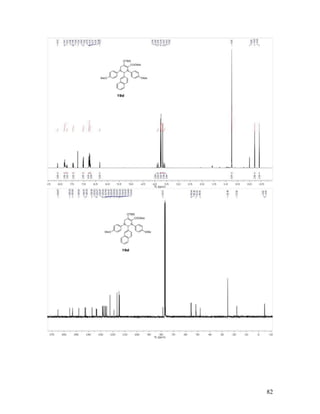
Methyl 2-(4-Methoxyphenyl)-2-[(4-methoxyphenyl)imino]acetate (17i).
Reaction between 4-methoxyphenyldiazoacetate 16f and p-anisyl azide 6a followed by
chromatographic purification gave 17i as a single geometric isomer in 90% isolated yield.
Yellow solid, mp = 93-94 °C; TLC Rf = 0.3 (4:1 hexanes/EtOAc); 1
H and 13
C NMR
spectral data is in accordance with the literature;48
IR (neat) 1729, 1605 cm-1
; HRMS
(ESI) m/z calculated for C17H18NO4 [M+H]+
300.1236, found: 300.1228.
Methyl 2-(4-chlorophenyl)-2-[(4-methoxyphenyl)imino]acetate (17j). Reaction
between 4-chlorophenyldiazoacetate 16g and p-anisyl azide 6a followed by
chromatographic purification gave 17j as a mixture of geometric isomers (93:7) in 95%](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-29-320.jpg)
![30
overall yield. Green liquid; TLC Rf = 0.4 (4:1 hexanes/EtOAc); 1
H NMR (400 MHz,
CDCl3) major isomer δ 7.83 (d, J = 8.7 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 6.99 (d, J = 9.0
Hz, 2H), 6.91 (d, J = 9.0 Hz, 2H), 3.84 (s, 3H), 3.72 (s, 3H); 13
C NMR (100 MHz,
CDCl3) δ 165.8, 157.6, 157.5, 142.8, 137.7, 132.6, 129.1, 128.9, 121.2, 114.2, 55.4, 52.1;
IR (neat) 1730, 1502 cm-1
; HRMS (ESI) m/z calculated for C16H15ClNO3 [M+H]+
304.0740, found: 304.0740.
Methyl 2-(4-Methoxyphenyl)imino-2-(4-nitrophenyl)acetate (17k). Reaction
between 4-nitrophenyldiazoacetate 16h and p-anisyl azide 6a followed by
chromatographic purification gave 17k as a single geometric isomer in 82% overall yield.
Orange solid, mp = 73-75 °C; TLC Rf = 0.3 (4:1 hexanes/EtOAc); 1
H NMR (400 MHz,
CDCl3) δ 8.30 (d, J = 8.7 Hz, 2H), 8.04 (d, J = 8.7 Hz, 2H), 7.00 (d, J = 8.9 Hz, 2H), 6.91
(d, J = 8.9 Hz, 2H), 3.83 (s, 3H), 3.74 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 165.4,
158.2, 156.0, 149.3, 142.2, 139.6, 128.7, 123.8, 121.5, 114.4, 55.4, 52.4; IR (neat) 1722,
1602, 1517 cm-1
; HRMS (ESI) m/z calculated for C16H15N2O5 [M+H]+
315.0981, found:
315.0976.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-30-320.jpg)
![31
Methyl 2-(4-Methoxyphenyl)imino-2-(2-naphthyl)acetate (17l). Reaction
between 2-napthyldiazoacetate compound 16i and p-anisyl azide 6a followed by
chromatographic purification gave 17l as a mixture of geometric isomers (94:6) in 96%
overall yield. Yellow solid, mp = 78-81 °C; TLC Rf = 0.4 (4:1 hexanes/EtOAc); 1
H and
13
C NMR spectral data are in accordance with the literature;48
IR (neat) 1734, 1609 cm-1
;
HRMS (ESI) m/z calculated for C20H18NO3 [M+H]+
320.1287, found: 320.1282.
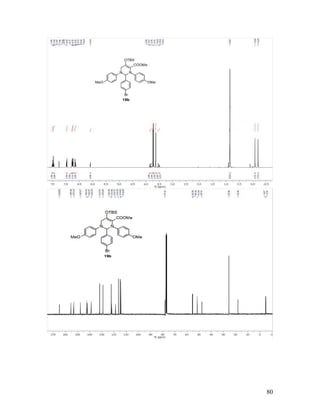
Dimethyl 2-(4-Methoxyphenyl)iminomalonate (17m). Reaction between
diazomalonate ester 16b and p-anisyl azide 6a followed by chromatographic purification
gave 17m in 60% yield. Red oil; TLC Rf = 0.45 (2:1 hexanes/EtOAc); 1
H NMR (400
MHz, CDCl3) δ 7.09 (d, J = 9.0 Hz, 2H), 6.89 (d, J = 9.0 Hz, 2H), 3.97 (s, 3H), 3.82 (s,
3H), 3.77 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 163.9, 161.8, 159.5, 148.9, 139.8,
122.8, 114.4, 55.4, 53.5, 52.6; IR (neat) 1739, 1723 cm-1
; HRMS (ESI) m/z calculated for
C12H14NO5 [M+H]+
252.0872, found: 252.0884.
Methyl 2-(4-Methoxyphenyl)imino-3-oxo-4-(3-oxocyclohexyl)butanoate
(17n). Diazoacetoacetate compound 16j was synthesized following a known protocol.47](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-31-320.jpg)
![32
The reaction of 16j with p-anisyl azide 6a followed by chromatographic purification gave
17n as a single geometric isomer in 65% yield. Dark red liquid; TLC Rf = 0.35 (2:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 9.0 Hz, 2H), 6.90 (d, J = 9.0
Hz, 2H), 3.83 (s, 3H), 3.78 (s, 3H), 3.02 (qd, J = 16.6, 6.6 Hz, 2H), 2.54 – 2.35 (comp,
3H), 2.34 – 2.21 (m, 1H), 2.21 – 2.11 (m, 1H), 2.11 – 1.93 (comp, 2H), 1.79 – 1.65 (m,
1H), 1.52 – 1.40 (m, 1H); 13
C NMR (100 MHz, CDCl3) δ 210.5, 197.5, 165.1, 159.9,
154.2, 139.3, 123.5, 114.5, 55.5, 52.4, 47.6, 42.7, 41.2, 34.7, 31.0, 24.9; IR (neat) 1735,
1705, 1502 cm-1
; HRMS (ESI) m/z calculated for C18H22NO5 [M+H]+
332.1498, found:
332.1473.
Methyl 3-(tert-Butyldimethylsilyl)oxy-2-[(4-methoxyphenyl)imino]but-3-
enoate (17o). Reaction between silyl enol diazoacetate 16d and p-anisyl azide 6a
followed by chromatographic purification gave 17o as a single geometric isomer in 40%
yield. Yellow solid; TLC Rf = 0.35 (10:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3)
δ 6.89 (d, J = 9.1 Hz, 2H), 6.84 (d, J = 9.1 Hz, 2H), 5.09 (d, J = 1.9 Hz, 1H), 4.90 (d, J =
1.9 Hz, 1H), 3.79 (s, 3H), 3.62 (s, 3H), 0.95 (s, 9H), 0.23 (s, 6H); 13
C NMR (100 MHz,
CDCl3) δ 165.5, 157.5, 157.2, 152.3, 142.2, 121.2, 114.1, 101.5, 55.4, 51.9, 25.6, 18.3, -
4.7; IR (neat) 1735, 1609, 1502 cm-1
; HRMS (ESI) m/z calculated for C18H28NO4Si
[M+H]+
350.1788, found: 350.1778.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-32-320.jpg)
![33
Chapter 2: Synthesis of 1,2,3,4-Tetrahydropyrimidines from Novel
[4+2] Cycloadditions Between an Azadiene and Aldimines
2.1 Background
In the course of preparing several α-iminoesters, we attempted to synthesize
azadiene 17o from silyl enoldiazoacetate 1b (Scheme 19). We envisioned that azadiene
17o could participate in hetero-Diels-Alder reactions with dienophiles. Though the
reaction was successful, azadiene 17o was isolated in only 40% yield.
Scheme 19. Metal-catalyzed synthesis of azadiene 17o from 16d
An alternative synthesis of this compound was accomplished by treating imine 17b with
TBSOTf and EtN3 (Scheme 20). No column chromatography was necessary for this step.
Scheme 20. Alternative synthesis of azadiene 17o from 17b
Now, with an efficient route for the large-scale synthesis of azadiene 17o, we
were delighted to discover a [4+2] cycloaddition reaction between 17o and aldimines 18
to yield highly functionalized 1,2,3,4-tetrahydropyrimidines 19 (Scheme 21). To our](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-33-320.jpg)
![34
knowledge, this Lewis acid catalyzed [4+2] cycloaddition reaction is the first example of
the aza-Diels-Alder reaction49,50
with imines taking the role of both the diene and
dienophile.
2.2 Substrate Scope
Under optimized conditions (see experimental section), the scope of this Lewis
acid-catalyzed [4+2] reaction was examined.
entry Ar 19 yield (%)
1 C6H5 19a 91
2 4-BrC6H5 19b 88
3 4-NO2C6H5 19c 90
4 2-naphthyl 19d 82
5 4-MeC6H5 19e 82
6 2-ClC6H4 19f 75
7 4-MeOC6H4 19g 72
Table 3. Synthesis of 1,2,3,4-tetrahydropyrimidines from novel [4+2] reaction
Hence, this methodology allows for the synthesis of novel functionalized
pyrimidine derivatives, which could be biologically active. Tetrahydropyrimidines are a
class of molecules that exhibit known antimicrobial51
and anti-inflammatory52
properties.
While compounds 19 could be derivatized and investigated for potential bioactivity, we
hope that other research groups will discover the potential of the metal-catalyzed [4+2]
cycloaddition between two imines and produce similar molecules with diverse](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-34-320.jpg)


![37
Methyl 5-(tert-Butyldimethylsilyl)oxy-1,3-bis(4-methoxyphenyl)-2-phenyl-
1,2,3,4-tetrahydropyrimidine-6-carboxylate (19a). Reaction between 17o and (E)-N-
(4-methoxyphenyl)-1-phenylmethanimine 18a followed by chromatographic purification
gave 19a in 91% yield. Light green oil; TLC Rf = 0.3 (10:1 hexanes/EtOAc); 1
H NMR
(400 MHz, CDCl3) δ 7.65 – 7.58 (comp, 2H), 7.37 – 7.30 (comp, 2H), 7.30 – 7.25 (m,
1H), 6.99 (d, J = 9.0 Hz, 2H), 6.76 (d, J = 7.5 Hz, 2H), 6.74 (d, J = 7.5 Hz, 2H), 6.66 (d,
J = 9.0 Hz, 2H), 6.19 (s, 1H), 3.85 (d, J = 17.5 Hz, 1H), 3.74 (s, 3H), 3.71 (s, 3H), 3.64
(s, 3H), 3.56 (d, J = 17.5 Hz, 1H), 0.84 (s, 9H), -0.14 (s, 3H), -0.28 (s, 3H); 13
C NMR
(100 MHz, CDCl3) δ 165.0, 155.4, 152.9, 147.8, 142.7, 142.3, 139.5, 128.7, 127.9, 127.1,
122.4, 118.9, 116.1, 114.8, 114.4, 78.5, 55.6, 55.4, 51.6, 47.8, 25.6, 18.0, -4.8, -5.0; IR
(neat) 1718, 1508 cm-1
; HRMS (ESI) m/z calculated for C32H41N2O5Si [M+H]+
561.2785,
found: 561.2783.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-37-320.jpg)
![38
Methyl 2-(4-Bromophenyl)-5-(tert-butyldimethylsilyl)oxy-1,3-bis(4-
methoxyphenyl)-1,2,3,4-tetrahydropyrimidine-6-carboxylate (19b). Reaction between
17o and (E)-1-(4-bromophenyl)-N-(4-methoxyphenyl)methanimine 18b followed by
chromatographic purification gave 19b in 88% yield. Light green oil; TLC Rf = 0.3 (10:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.8, 2H), 7.46 (d, J = 8.8 Hz,
2H), 6.97 (d, J = 9.0 Hz, 2H), 6.76 (d, J = 8.0 Hz, 2H), 6.74 (d, J = 8.0 Hz, 2H), 6.65 (d,
J = 9.0 Hz, 2H), 6.09 (s, 1H), 3.84 (d, J = 17.5 Hz, 1H), 3.75 (s, 3H), 3.72 (s, 3H), 3.64
(s, 3H), 3.52 (d, J = 17.5 Hz, 1H), 0.86 (s, 9H), -0.08 (s, 3H), -0.20 (s, 3H); 13
C NMR
(100 MHz, CDCl3) δ 164.9, 155.49, 153.1, 147.7, 142.4, 142.0, 138.7, 131.8, 129.1,
122.4, 121.9, 118.7, 116.4, 114.8, 114.4, 78.1, 55.6, 55.4, 51.6, 47.7, 25.6, 18.1, -4.7, -
4.8; IR (neat) 1718, 1507 cm-1
; HRMS (ESI) m/z calculated for C32H40BrN2O5Si [M+H]+
639.1890, found: 639.1899.
Methyl 5-(tert-Butyldimethylsilyl)oxy-1,3-bis(4-methoxyphenyl)-2-(4-
nitrophenyl)-1,2,3,4-tetrahydropyrimidine-6-carboxylate (19c). Reaction between 17o
and (E)-N-(4-methoxyphenyl)-1-(4-nitrophenyl)methanimine 18c followed by
chromatographic purification gave 19c in 90% yield. Orange solid; TLC Rf = 0.25 (5:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.9 Hz, 2H), 7.83 (dd, J =
9.0, 0.8 Hz, 2H), 6.99 (d, J = 9.0 Hz, 2H), 6.78 (d, J = 7.1 Hz, 2H), 6.76 (d, J = 7.1 Hz,](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-38-320.jpg)
![39
2H), 6.67 (d, J = 9.0 Hz, 2H), 6.14 (s, 1H), 3.87 (d, J = 17.6 Hz, 1H), 3.76 (s, 3H), 3.73
(s, 3H), 3.65 (s, 3H), 3.51 (d, J = 17.6 Hz, 1H), 0.84 (s, 9H), -0.09 (s, 3H), -0.21 (s, 3H);
13
C NMR (100 MHz, CDCl3) δ 164.7, 155.8, 153.6, 147.8, 147.5, 147.5, 142.1, 141.6,
128.4, 123.9, 122.5, 118.9, 116.7, 115.0, 114.5, 78.3, 55.6, 55.4, 51.8, 47.8, 25.5, 18.1, -
4.5, -4.6; IR (neat) 1726, 1508 cm-1
; HRMS (ESI) m/z calculated for C32H40N3O7Si
[M+H]+
606.2636, found: 606.2642.
Methyl 5-(tert-Butyldimethylsilyl)oxy-1,3-bis(4-methoxyphenyl)-2-
(naphthalen-2-yl)-1,2,3,4-tetrahydropyrimidine-6-carboxylate (19d). Reaction
between 17o and (E)-N-(4-methoxyphenyl)-1-(naphthalen-2-yl)methanimine 18d
followed by chromatographic purification gave 19d in 82% yield. Light yellow liquid;
TLC Rf = 0.3 (8:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 8.11 (s, 1H), 7.85 –
7.77 (comp, 3H), 7.71 (dd, J = 8.6, 1.7 Hz, 1H), 7.49 – 7.42 (comp, 2H), 7.03 (d, J = 9.0
Hz, 2H), 6.81 – 6.75 (comp, 4H), 6.73 (d, J = 9.4 Hz, 2H), 6.33 (s, 1H), 3.88 (d, J = 17.5
Hz, 1H), 3.76 (s, 3H), 3.74 (s, 3H), 3.67 (s, 3H), 3.60 (d, J = 17.5 Hz, 1H), 0.75 (s, 9H), -
0.22 (s, 3H), -0.41 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 165.0, 155.3, 153.1, 147.9,
142.8, 142.3, 137.0, 133.5, 133.2, 128.5, 128.3, 127.4, 126.5, 125.9, 125.9, 125.0, 122.2,
118.9, 116.5, 114.8, 114.4, 78.7, 55.6, 55.4, 51.6, 48.0, 25.5, 18.0, -4.9, -5.0; IR (neat)](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-39-320.jpg)
![40
1718, 1507 cm-1
; HRMS (ESI) m/z calculated for C36H43N2O5Si [M+H]+
611.2941,
found: 611.2916.
Methyl 5-(tert-Butyldimethylsilyl)oxy-1,3-bis(4-methoxyphenyl)-2-(p-tolyl)-
1,2,3,4-tetrahydropyrimidine-6-carboxylate (19e). Reaction between 17o and (E)-N-
(4-methoxyphenyl)-1-(p-tolyl)methanimine 18e followed by chromatographic
purification gave 19e in 82% yield. Green liquid; TLC Rf = 0.35 (8:1 hexanes/EtOAc);
1
H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 7.9 Hz, 2H), 7.13 (d, J = 7.9 Hz, 2H), 6.98 (d,
J = 9.0 Hz, 2H), 6.76 (d, J = 6.9 Hz, 2H), 6.74 (d, J = 6.9 Hz, 2H), 6.65 (d, J = 9.0 Hz,
2H), 6.16 (s, 1H), 3.83 (d, J = 17.4 Hz, 1H), 3.74 (s, 3H), 3.72 (s, 3H), 3.63 (s, 3H), 3.55
(d, J = 17.4 Hz, 1H), 2.33 (s, 3H), 0.85 (s, 9H), -0.12 (s, 3H), -0.25 (s, 3H); 13
C NMR
(100 MHz, CDCl3) δ 165.0, 155.3, 152.8, 147.9, 142.8, 142.4, 137.5, 136.4, 129.3, 127.0,
122.3, 118.8, 116.1, 114.8, 114.4, 78.4, 55.6, 55.4, 51.6, 47.8, 25.6, 21.0, 18.1, -4.8, -4.9;
IR (neat) 1722, 1506 cm-1
; HRMS (ESI) m/z calculated for C33H43N2O5Si [M+H]+
575.2941, found: 575.2955.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-40-320.jpg)
![41
Methyl 5-(tert-Butyldimethylsilyl)oxy-2-(2-chlorophenyl)-1,3-bis(4-
methoxyphenyl)-1,2,3,4-tetrahydropyrimidine-6-carboxylate (19f). Reaction between
17o and (E)-1-(2-chlorophenyl)-N-(4-methoxyphenyl)methanimine 18f followed by
chromatographic purification gave 19f in 75% yield. Colorless liquid; TLC Rf = 0.35 (8:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.76 – 7.70 (m, 1H), 7.47 – 7.41 (m,
1H), 7.30 – 7.25 (comp, 2H), 6.81 (d, J = 9.1 Hz, 2H), 6.78 (d, J = 9.1 Hz, 2H), 6.71 –
6.63 (comp, 4H), 6.15 (s, 1H), 3.80 (d, J = 17.9 Hz, 1H), 3.71 (s, 3H), 3.70 (s, 3H), 3.60
(s, 3H), 3.50 (d, J = 17.9 Hz, 1H), 0.92 (s, 9H), 0.08 (s, 3H), 0.03 (s, 3H); 13
C NMR (100
MHz, CDCl3) δ 164.8, 154.9, 153.6, 145.6, 143.0, 142.1, 136.5, 133.4, 130.5, 129.8,
129.2, 126.7, 121.4, 119.5, 118.5, 114.3, 114.2, 77.4, 55.5, 55.4, 51.6, 48.2, 25.6, 18.1, -
3.8, -4.2; IR (neat) 1721, 1506 cm-1
; HRMS (ESI) m/z calculated for C32H40ClN2O5Si
[M+H]+
595.2395, found: 595.2396.
Methyl 5-(tert-Butyldimethylsilyl)oxy-1,2,3-tris(4-methoxyphenyl)-1,2,3,4-
tetrahydropyrimidine-6-carboxylate (19g). Reaction between 17o and (E)-N,1-bis(4-](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-41-320.jpg)
![42
methoxyphenyl)methanimine 18g followed by chromatographic purification gave 19g in
72% yield. Light green liquid; TLC Rf = 0.3 (6:1 hexanes/EtOAc); 1
H NMR (400 MHz,
CDCl3) δ 7.52 (d, J = 8.8 Hz, 2H), 6.98 (d, J = 8.9 Hz, 2H), 6.87 (d, J = 8.8 Hz, 2H), 6.78
– 6.72 (comp, 4H), 6.66 (d, J = 9.1 Hz, 2H), 6.14 (s, 1H), 3.79 (s, 3H), 3.74 (s, 3H), 3.72
(s, 3H), 3.64 (s, 3H), 0.86 (s, 9H), -0.10 (s, 3H), -0.22 (s, 3H); 13
C NMR (100 MHz,
CDCl3) δ 165.0, 159.4, 155.3, 152.9, 147.8, 142.7, 142.3, 131.5, 128.3, 122.3, 118.7,
116.2, 114.8, 114.3, 114.0, 78.2, 55.6, 55.4, 55.4, 51.6, 47.7, 25.6, 18.1, -4.7, -4.9; IR
(neat) 1721, 1505 cm-1
; HRMS (ESI) m/z calculated for C33H43N2O6Si [M+H]+
591.2890,
found: 591.2880.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-42-320.jpg)
![43
Chapter 3: Synthesis of β-Lactams by [2+2] Cycloadditions between
Ketene and Imines
3.1 Background
During our investigation of the substrate scope of the RhII
-catalyzed conversion of
diazo compounds to imines, we noticed that diazoacetoacetate enone 16k failed to form
the corresponding imine product 21. TLC analysis of the reaction mixture was messy,
and a significant amount of 6a was present on the plate, indicating that 6a had failed to
react with 16k (Scheme 21). Furthermore, 1
H NMR of the reaction mixture revealed a
significant amount of p-anisyl azide left over, corroborating the same hypothesis.
Scheme 21. Diazoacetoacetate enone 16k fails to react with 6a to form imine 21
3.2 Metal-Catalyzed Wolff Rearrangement
While the failure of 16k was an initial disappointment for us, we quickly
discovered the problem. When ~10 mg compound 16k was dissolved in an NMR tube
containing CDCl3 and a few milligrams of Rh2(OAc)4, and the NMR tube was heated at
60 °C for 1 hour, bubbles evolved from the bottom of the tube. Immediately, we
performed TLC analysis on this solution and observed a large streak. However, after
taking a 1
H NMR of the unpurified mixture, we noticed only one major compound in
solution, which we identified to be ketene 22 (Scheme 22). The methoxy, vinyl, and](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-43-320.jpg)
![44
phenyl protons of the ketene were clearly observed in the NMR spectrum, with little
impurities present and no 16k visible. Hence, a transition metal-catalyzed Wolff
rearrangement53-56
had occurred to generate ketene 22 in vitro.
Scheme 22. Diazoacetoacetate enone 16k undergoes Wolff Rearrangement
In order to verify our hypothesis that a true ketene was being formed within the
NMR tube, we added crystals of imine 17e to the NMR tube. Immediately afterwards, we
retook a 1
H NMR spectrum. The ketene 22 had completely disappeared and was replaced
by new signals. After purification of the mixture, β-lactam 23a was isolated and
characterized (Scheme 23).
Scheme 23. A [2+2] cycloaddition between ketene 22 and imine 17e formed a β-
lactam product with high diastereoselectivity
3.3 Staudinger Synthesis
We were impressed by the high diastereomeric ratio of compound 23a formed in
Scheme 23, which led us to develop an optimized method for the synthesis of the β-](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-44-320.jpg)
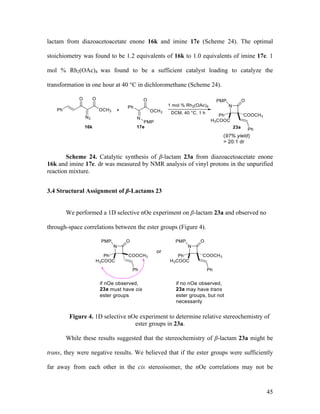


![48
hours of stirring at either room temperature or 40 °C, the β-lactam products were cleanly
generated in near-quantitative yields (Table 5).
entry R 23 yield (%)
dr
(anti:syn)
1 H 23a 99 > 20:1
2 OCH3 23c 99 > 20:1
3 Cl 23g 99 > 20:1
Table 5. Substrate scope for the one-pot synthesis of β-lactams 23
To our knowledge, this reaction represents the first case of a mixture of two diazo
compounds being simultaneously added to a solution containing a dirhodium catalyst.
The catalytic reaction of diazo compounds 16 with azide 6a occurs before the Wolff
rearrangement to form intermediate imines 17 in a fast step. Then, ketene 22 is generated
in situ, which undergoes a [2+2] cycloaddition with 17 to form β-lactams 23. Hence, the
reagents react in a tightly orchestrated manner.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-48-320.jpg)

![50
General Procedure for the Synthesis of β-Lactams 23. To a flame dried 4-dram
vial equipped with a magnetic stirbar was added phenyl diazoacetoacetate enone 16k
(0.43 mmol, 1.2 equiv), imine 17 (0.36 mmol, 1.0 equiv), and 2 mL of DCM. After
dissolution, Rh2(OAc)4 (1.0 mol %) was added and the solution was sealed with a septum
and N2 balloon. The reaction mixture was stirred for 1 h at 40 °C. Upon completion of the
reaction, the unpurified mixture was analyzed by 1
H NMR spectroscopy for
diastereoselectivity, and then chromatographed to afford β-lactams 23.
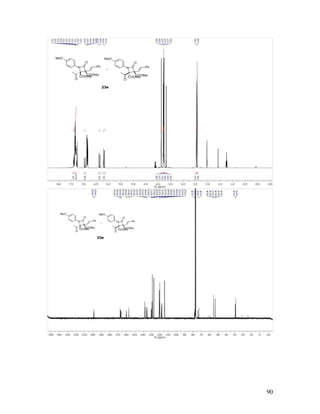
Dimethyl 1-(4-Methoxyphenyl)-4-oxo-2-phenyl-3-[(E)-styryl]azetidine-2,3-di-
carboxylate (23a). Reaction between phenyldiazoacetoacetate enone 16k and diaryl
imine 17e followed by chromatographic purification gave 23a in 97% yield, with > 20:1
dr. Recrystallized from DCM/hexanes as colorless crystals, mp = 170-174 °C; TLC Rf =
0.3 (4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.75 – 7.68 (m, 2H), 7.47 – 7.38
(comp, 4H), 7.38 – 7.24 (comp, 6H), 6.97 (d, J = 16.4 Hz, 1H), 6.84 (d, J = 9.1 Hz, 2H),
6.56 (d, J = 16.4 Hz, 1H), 3.79 (s, 3H), 3.61 (s, 3H), 3.18 (s, 3H); 13
C NMR (100 MHz,
CDCl3) δ 167.8, 166.3, 160.4, 156.3, 135.8, 135.1, 131.0, 130.6, 129.0, 128.7, 128.5,
128.2, 128.0, 126.8, 119.7, 119.0, 113.9, 76.3, 75.0, 55.4, 52.9, 52.3; IR (neat) 1764,](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-50-320.jpg)
![51
1737, 1510 cm-1
; HRMS (ESI) m/z calculated for C28H26NO6 [M+H]+
472.1760, found:
472.1768.
Dimethyl 1-(4-Nitrophenyl)-4-oxo-2-phenyl-3-[(E)-styryl]azetidine-2,3-
dicarboxylate (23b). Reaction between phenyldiazoacetoacetate enone 16k and imine
17g followed by chromatographic purification gave 23b in 95% yield, with > 20:1 dr.
Recrystallized from DCM/hexanes as pale yellow solid, mp = 143-144 °C; TLC Rf = 0.3
(4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 9.4 Hz, 2H), 7.71 –
7.64 (comp, 2H), 7.60 (d, J = 9.4 Hz, 2H), 7.46 – 7.27 (comp, 8H), 6.93 (d, J = 16.4 Hz,
1H), 6.55 (d, J = 16.4 Hz, 1H), 3.66 (s, 3H), 3.21 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ
167.0, 165.6, 161.4, 143.8, 142.1, 135.4, 135.3, 129.9, 129.6, 128.8, 128.8, 128.5, 127.8,
126.9, 124.7, 118.6, 118.4, 75.8, 53.3, 52.5. IR (neat) 1776, 1740 cm-1
; HRMS (ESI) m/z
calculated for C27H23N2O7 [M+H]+
487.1505, found: 487.1518.
Dimethyl 1,2-bis(4-Methoxyphenyl)-4-oxo-3-[(E)-styryl]azetidine-2,3-
dicarboxylate (23c). Reaction between phenyldiazoacetoacetate enone 16k and diaryl](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-51-320.jpg)
![52
imine 17i followed by chromatographic purification gave 23c in 81% yield, with > 20:1
dr. Recrystallized from DCM/hexanes as colorless crystals, mp = 181-184 °C; TLC Rf =
0.3 (3:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 9.1 Hz, 2H), 7.42 –
7.38 (comp, 2H), 7.41 (d, J = 9.1 Hz, 2H), 7.36 – 7.23 (comp, 3H), 6.95 (d, J = 16.4 Hz,
1H), 6.85 (d, J = 7.5 Hz, 2H), 6.83 (d, J = 7.5 Hz, 2H), 6.54 (d, J = 16.4 Hz, 1H), 3.79 (s,
3H), 3.78 (s, 3H), 3.60 (s, 3H), 3.25 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 168.0, 166.4,
160.5, 160.0, 156.3, 135.9, 134.9, 130.6, 129.6, 128.7, 128.5, 126.8, 122.6, 119.8, 119.2,
113.9, 113.4, 76.3, 74.9, 55.4, 55.2, 52.8, 52.4; IR (neat) 1762, 1739, 1510 cm-1
; HRMS
(ESI) m/z calculated for C29H28NO7 [M+H]+
502.1866, found: 502.1864.
Dimethyl 1-(4-Methoxyphenyl)-2-(4-Nitrophenyl)-4-oxo-3-((E)-
styryl)azetidine-2,3-dicarboxylate (23d). Reaction between phenyldiazoacetoacetate
enone 16k and diaryl imine 17k followed by chromatographic purification gave 23d in
89% yield, with 15:1 dr. Recrystallized from DCM/hexanes as pale yellow solid, mp =
160-161 °C; TLC Rf = 0.25 (4:1 hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) of major
isomer δ 8.19 (d, J = 9.1 Hz, 2H), 7.95 (d, J = 9.2 Hz, 2H), 7.47 – 7.40 (m, 2H), 7.39 –
7.28 (comp, 5H), 7.00 (d, J = 16.4 Hz, 1H), 6.87 (d, J = 9.1 Hz, 2H), 6.51 (d, J = 16.4 Hz,
1H), 3.80 (s, 3H), 3.61 (s, 3H), 3.24 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 167.2, 165.9,
159.7, 156.7, 148.0, 138.4, 136.2, 135.5, 130.0, 129.6, 128.9, 128.8, 126.9, 122.9, 119.4,
117.8, 114.3, 76.7, 74.1, 55.5, 53.4, 52.6; IR (neat) 1768, 1738, 1511 cm-1
; HRMS (ESI)
m/z calculated for C28H25N2O8 [M+H]+
517.1611, found: 517.1601.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-52-320.jpg)
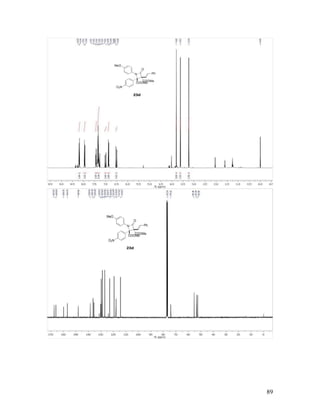
![53
Dimethyl (E)-2-Acetyl-1-(4-Methoxyphenyl)-4-oxo-3-styrylazetidine-2,3-di-
carboxylate (23e). Reaction between phenyldiazoacetoacetate enone 16k and imine 17b
followed by chromatographic purification gave 23e in 93% yield, with 1.38:1 dr. White
solid, mp not determined due to presence of both diastereomers; TLC Rf = 0.2 (3:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ values for major isomer: 6.18 (d, J =
16.2 Hz, 1H), 3.88 (s, 3H), 3.78 (s, 3H), 3.77 (s, 3H), 2.43 (s, 3H) (aromatic resonances
of both stereoisomers are obscured by overlap; vinyl resonance of major isomer obscured
by overlap with other aromatic signals); δ values for minor isomer: 6.95 (d, J = 16.3 Hz,
1H), 6.36 (d, J = 16.3 Hz, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.68 (s, 3H), 2.47 (s, 3H). 13
C
NMR (100 MHz, CDCl3) δ values for both stereoisomers: 198.9, 198.6, 167.3, 167.0,
166.8, 166.2, 159.9, 159.7, 157.0, 157.0, 137.9, 136.5, 135.3, 135.0, 130.1, 129.8, 129.1,
128.9, 128.7, 128.7, 127.0, 126.9, 120.5, 119.9, 118.0, 117.4, 114.2, 114.1, 78.4, 77.9,
74.2, 73.0, 55.5, 55.4, 53.5, 53.5, 53.4, 53.3, 29.4, 28.9; IR (neat) 1767, 1739, 1728, 1511
cm-1
; HRMS (ESI) m/z calculated for C24H24NO7 [M+H]+
438.1553, found: 438.1558.
Dimethyl 2-(1-(tert-Butyldimethylsilyl)oxyvinyl)-1-(4-Methoxyphenyl)-4-oxo-
3-[(E)-styryl]azetidine-2,3-dicarboxylate (23f). Reaction between](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-53-320.jpg)
![54
phenyldiazoacetoacetate enone 16k and imine 17o followed by chromatographic
purification gave 23f in 85% yield, with >20:1 dr. Recrystallized from DCM/hexanes as
colorless crystals, mp = 145-150 °C; TLC Rf = 0.3 (4:1 hexanes/EtOAc); 1
H NMR (400
MHz, CDCl3) δ 7.54 (d, J = 9.1 Hz, 2H), 7.39 – 7.34 (m, 2H), 7.33 – 7.23 (m, 3H), 6.93
(d, J = 16.2 Hz, 1H), 6.83 (d, J = 9.1 Hz, 2H), 6.28 (d, J = 16.2 Hz, 1H), 4.93 (d, J = 3.1
Hz, 1H), 4.61 (d, J = 3.1 Hz, 1H), 3.79 (s, 3H), 3.78 (s, 3H), 3.69 (s, 3H), 0.74 (s, 9H),
0.09 (s, 3H), -0.08 (s, 3H); 13
C NMR (100 MHz, CDCl3) δ 167.2, 166.4, 160.4, 156.3,
149.6, 135.6, 135.1, 131.0, 128.6, 128.5, 126.8, 120.5, 119.4, 113.7, 96.3, 75.7, 71.6,
55.5, 52.7, 52.7, 25.3, 17.9, -5.0, -5.7; IR (neat) 1764, 1739, 1509 cm-1
; HRMS (ESI) m/z
calculated for C30H38NO7Si [M+H]+
552.2418, found: 552.2405.
Procedure for the One-Pot, Multicomponent Synthesis of β-Lactams 23. To a
flame dried 4 dram vial equipped with a magnetic stirbar was added p-anisyl azide 6a
(54.0 mg, 0.362 mmol, 1.0 equiv), 1 mL of DCM, and Rh2(OAc)4 (1.8 mg, 1.0 mol %,
0.0036 mmol). The solution was then sealed with a septum and N2 balloon and stirred at
40 °C for 23a and 23c, or at room temperature for 23g. A mixture of 16(c,g, or f) (0.434
mmol, 1.2 equiv) and phenyl diazoacetoacetate enone 16k (100.0 mg, 0.434 mmol, 1.2
equiv) in 2 mL of DCM was injected into the vial over the course of 2 h. The reaction
mixture was stirred for an additional 1 h at the given temperature. Upon completion of
the reaction, the unpurified mixture was chromatographed to afford β-lactam 23.](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-54-320.jpg)
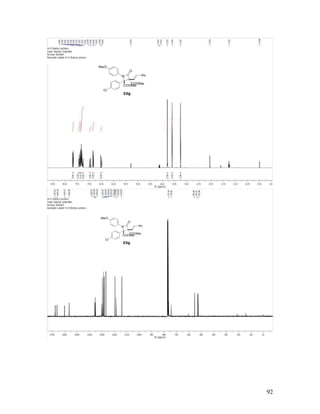
![55
Dimethyl 2-(4-Chlorophenyl)-1-(4-methoxyphenyl)-4-oxo-3-([E]-
styryl)azetidine-2,3-dicarboxylate (23g). Reaction between diazo compound 16g,
phenyldiazoacetoacetate enone 16k and p-anisyl azide 6a at room temperature, followed
by chromatographic purification gave 23g in 99% yield, with >20:1 dr. Recrystallized
from DCM/hexanes as colorless crystals, mp = 194-196 °C; TLC Rf = 0.4 (3:1
hexanes/EtOAc); 1
H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 8.8 Hz, 2H), 7.42 (d, J = 7.0
Hz, 2H), 7.37 (d, J = 9.1 Hz, 2H), 7.35 – 7.25 (comp, 5H), 6.97 (d, J = 16.4 Hz, 1H), 6.85
(d, J = 9.1 Hz, 2H), 6.52 (d, J = 16.4 Hz, 1H), 3.79 (s, 3H), 3.60 (s, 3H), 3.25 (s, 3H); 13
C
NMR (100 MHz, CDCl3) δ 167.6, 166.2, 160.1, 156.5, 135.7, 135.4, 135.3, 130.3, 129.8,
129.6, 128.7, 128.6, 128.2, 126.8, 119.6, 118.6, 114.1, 76.3, 74.4, 55.4, 53.1, 52.4; IR
(neat) 1768, 1737, 1508 cm-1
; HRMS (ESI) m/z calculated for C28H25ClNO6 [M+H]+
506.1370, found: 506.1355.
X-ray structure of compound 23a:](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-55-320.jpg)

![57
Crystal size 0.16 × 0.20 × 0.47 mm
Crystal habit colorless prism
Crystal system monoclinic
Space group P21/c
Unit cell dimensions a = 8.3217(7) Å α = 90°
b = 14.4515(12) Å β = 100.2680(13)°
c = 20.1226(17) Å γ = 90°
Volume 2381.2(3) Å3
Z 4
Density (calculated) 1.315 Mg/cm3
Absorption coefficient 0.093 mm-1
F(000) 992
Table 2. Data collection and structure refinement for
UM2489.
Theta range for
data collection
1.75 to 30.00°
Index ranges
-11 ≤ h ≤ 11, -20 ≤ k
≤ 20, -28 ≤ l ≤ 28
Reflections
collected
38583
Independent
reflections
6952 [R(int) = 0.0215]
Coverage of
independent
reflections
100.0%
Max. and min.
transmission
0.9850 and 0.9150
Structure solution
technique
direct methods
Structure solution
program
ShelXS-97 (Sheldrick, 2008)
Refinement
method
Full-matrix least-squares on F2
Refinement
program
ShelXL-2012 (Sheldrick, 2012)
Function
minimized
Σ w(Fo
2
- Fc
2
)2
Data / restraints / 6952 / 11 / 425](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-57-320.jpg)
![58
parameters
Goodness-of-fit on
F2 1.000
Δ/σmax 0.001
Final R indices 5952 data; I>2σ(I)
R1 = 0.0399, wR2 =
0.0817
all data
R1 = 0.0476, wR2 =
0.0863
Weighting scheme
w=1/[σ2
(Fo
2
)+(0.0200P)2
+1.2330P] ,
P=(Fo
2
+2Fc
2
)/3
Largest diff. peak
and hole
0.342 and -0.175 eÅ-3
R.M.S. deviation
from mean
0.039 eÅ-3
Table 3. Atomic coordinates and equivalent isotropic
atomic displacement parameters (Å2
) for UM2489.
U(eq) is defined as one third of the trace of the orthogonalized Uij tensor.
x/a y/b z/c U(eq)
N1 0.13814(10) 0.73320(6) 0.00380(4) 0.02147(16)
C2 0.15288(12) 0.73633(7) 0.07795(5) 0.02098(18)
C3 0.34134(12) 0.70184(7) 0.08011(5) 0.02111(18)
C4 0.29521(12) 0.70643(7) 0.00270(5) 0.02192(19)
O4 0.36773(9) 0.69322(6) 0.95663(4) 0.02904(17)
C11 0.00108(12) 0.74475(7) 0.95163(5) 0.02146(18)
C12 0.85646(13) 0.78202(8) 0.96512(5) 0.0270(2)
C13 0.72002(13) 0.78936(8) 0.91399(6) 0.0284(2)
C14 0.72836(13) 0.75931(8) 0.84911(5) 0.0255(2)
C15 0.87450(13) 0.72314(8) 0.83547(5) 0.0258(2)
C16 0.01023(12) 0.71563(7) 0.88614(5) 0.02367(19)
O17 0.60044(10) 0.76080(7) 0.79586(4) 0.03414(19)
C17 0.44929(15) 0.79866(12) 0.80844(7) 0.0402(3)
C21 0.03266(12) 0.67290(7) 0.10457(5) 0.02186(19)
C22 0.96399(15) 0.69402(9) 0.16097(6) 0.0328(2)
C23 0.84858(16) 0.63542(10) 0.18087(7) 0.0386(3)
C24 0.80127(14) 0.55534(9) 0.14562(6) 0.0343(3)
C25 0.86878(15) 0.53331(9) 0.08961(6) 0.0328(2)](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-58-320.jpg)


![64
C4-C3-C38-O39 1.12(12) C2-C3-C38-O39 94.53(10)
O38-C38-O39-C39 4.33(16) C3-C38-O39-C39 -175.17(9)
Table 7. Anisotropic atomic displacement parameters
(Å2
) for UM2489.
The anisotropic atomic displacement factor exponent takes the form: -2π2
[
h2
a*2
U11 + ... + 2 h k a*
b*
U12 ]
U11 U22 U33 U23 U13 U12
N1 0.0212(4) 0.0247(4) 0.0189(4)
-
0.0018(3)
0.0046(3) 0.0001(3)
C2 0.0214(4) 0.0216(4) 0.0198(4)
-
0.0030(3)
0.0031(3) 0.0001(3)
C3 0.0206(4) 0.0206(4) 0.0225(5)
-
0.0011(4)
0.0049(3) -0.0012(3)
C4 0.0217(4) 0.0203(4) 0.0238(5)
-
0.0007(4)
0.0044(4) -0.0025(3)
O4 0.0261(4) 0.0365(4) 0.0265(4)
-
0.0008(3)
0.0099(3) 0.0001(3)
C11 0.0230(4) 0.0204(4) 0.0209(4) 0.0007(3) 0.0036(3) -0.0017(3)
C12 0.0270(5) 0.0322(5) 0.0220(5)
-
0.0022(4)
0.0052(4) 0.0038(4)
C13 0.0251(5) 0.0352(6) 0.0252(5) 0.0010(4) 0.0053(4) 0.0063(4)
C14 0.0257(5) 0.0283(5) 0.0217(5) 0.0048(4) 0.0025(4) 0.0000(4)
C15 0.0278(5) 0.0300(5) 0.0202(5) 0.0001(4) 0.0055(4) -0.0013(4)
C16 0.0237(5) 0.0249(5) 0.0233(5)
-
0.0002(4)
0.0066(4) -0.0005(4)
O17 0.0269(4) 0.0522(5) 0.0221(4) 0.0032(3) 0.0009(3) 0.0066(4)
C17 0.0271(6) 0.0651(9) 0.0280(6) 0.0102(6) 0.0036(5) 0.0094(6)
C21 0.0194(4) 0.0254(5) 0.0209(4)
-
0.0004(4)
0.0039(3) 0.0003(4)
C22 0.0315(5) 0.0398(6) 0.0294(6)
-
0.0091(5)
0.0120(4) -0.0043(5)
C23 0.0343(6) 0.0529(8) 0.0330(6)
-
0.0051(6)
0.0177(5) -0.0056(5)
C24 0.0258(5) 0.0434(7) 0.0349(6) 0.0062(5) 0.0085(4) -0.0060(5)
C25 0.0337(6) 0.0308(6) 0.0346(6)
-
0.0014(5)
0.0079(5) -0.0083(5)
C26 0.0299(5) 0.0281(5) 0.0261(5)
-
0.0041(4)
0.0094(4) -0.0041(4)
C27 0.0227(5) 0.0248(6) 0.0285(13) - 0.0057(13) 0.0003(6)](https://image.slidesharecdn.com/d567c9e3-cc90-4b6c-9d1c-c8feb97c62a3-151109005808-lva1-app6892/85/mandlerthesis2015draft4-64-320.jpg)