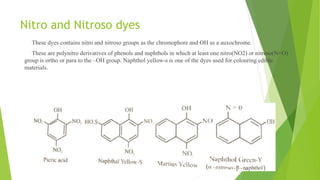
This document discusses the classification and properties of dyes. It outlines seven main classifications of dyes based on their chemical structure: 1) nitro and nitroso dyes, 2) azo dyes, 3) triphenyl methane dyes, 4) phthalein dyes, 5) indigoid and thioindigoid dyes, 6) antraquinone dyes, and 7) miscellaneous dyes. It then provides examples of azo dyes, including methyl orange. Methyl orange is described as an acidic azo dye containing a sulphonic acid group that makes it water soluble and allows it to be used as a dye and pH indicator that is orange in alkaline solutions and red