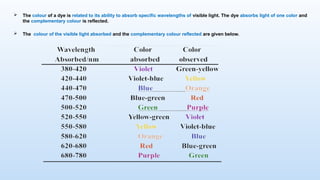
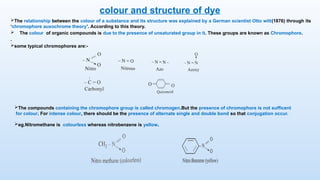
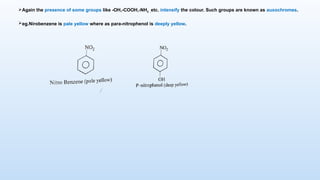
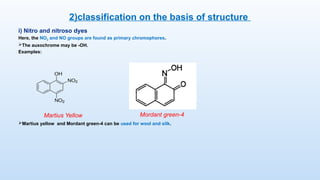
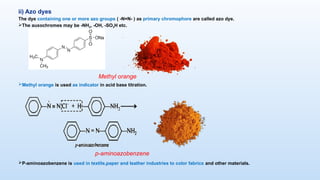
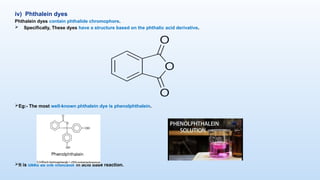
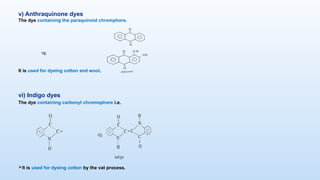
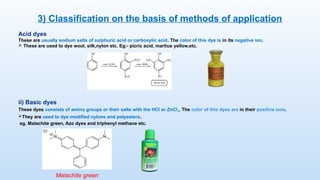
The document discusses the nature and use of dyes, which are chemical substances used to impart color to various materials. It differentiates between natural dyes, derived from plants and insects, and synthetic dyes, which dominate today’s market and are primarily made from coal tar. The document also covers the classification, properties, and advantages and disadvantages of dyes, particularly focusing on their environmental impact and potential health hazards.