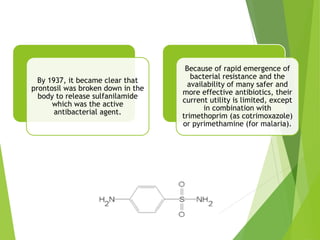
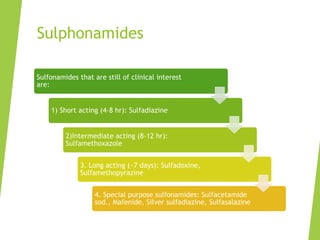
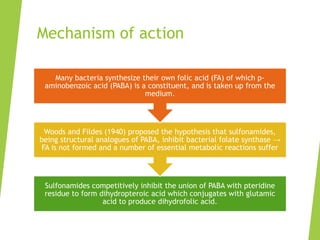
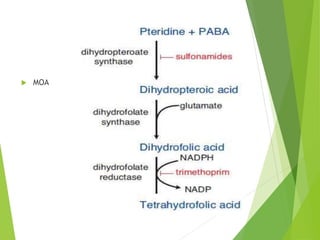
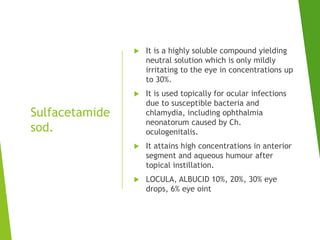
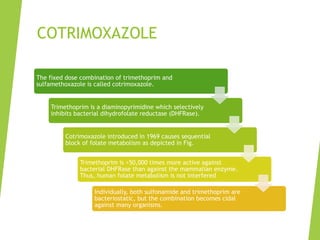
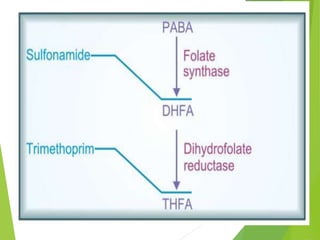
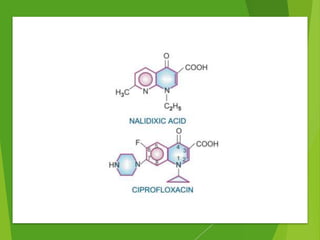
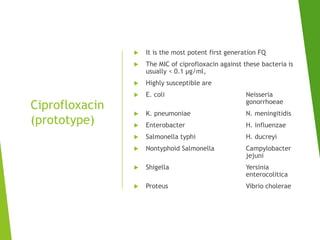
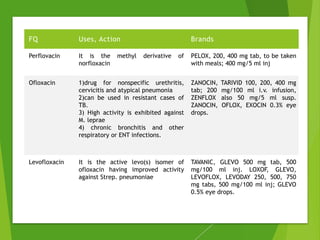
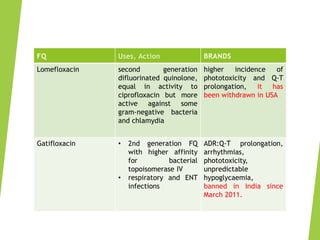
The document discusses sulfonamides, cotrimoxazole, and quinolones, detailing their historical significance, mechanism of action, clinical uses, and resistance patterns. It highlights the limitations of sulfonamides due to bacterial resistance and their combination use with trimethoprim as cotrimoxazole for various infections. Additionally, it covers the evolution of quinolones, particularly fluoroquinolones, their mechanisms, uses in treating gram-negative infections, and potential adverse effects.