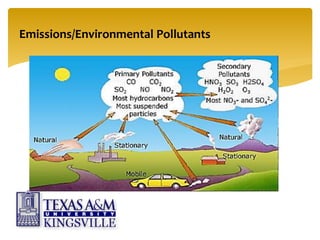
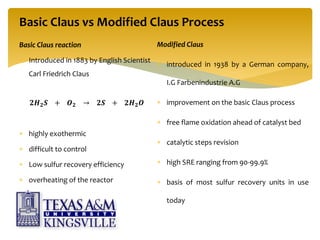
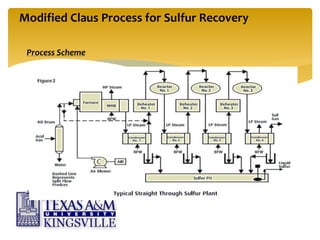
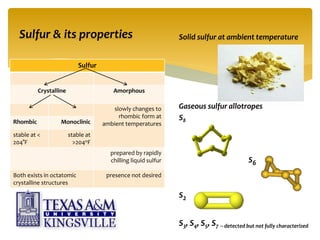
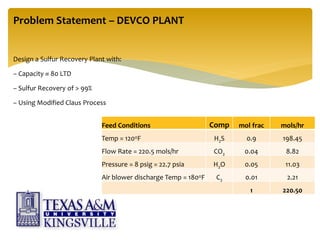
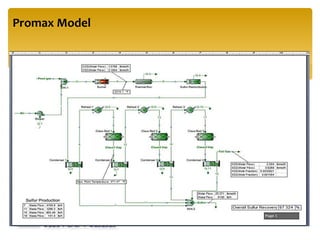
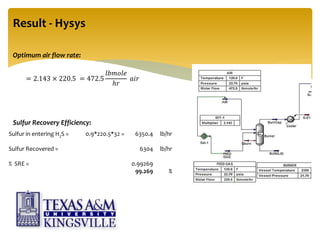
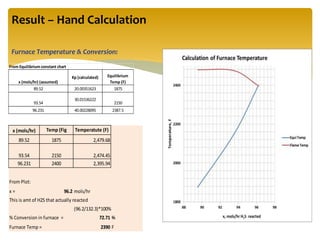
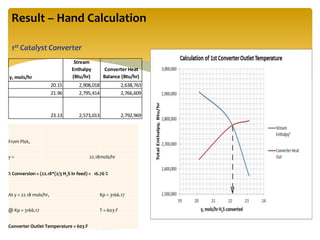
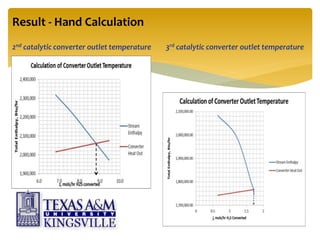
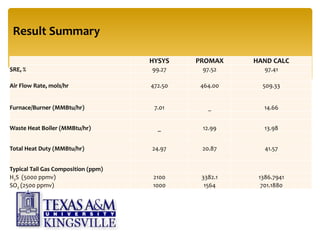
The document describes the modified Claus process for sulfur recovery. It discusses the basic Claus reaction and how the modified process improved on it with a free flame oxidation ahead of the catalyst bed and catalytic step revisions, allowing for higher sulfur recovery efficiencies of 90-99.9%. The key steps of the modified Claus process are presented as the combustion step and multiple catalytic steps. Process variations like the straight-through and split-flow configurations are described along with tail gas handling and other sulfur removal processes. Sample calculations are provided to determine the optimum operating parameters for a 80 long ton per day sulfur recovery unit using the modified Claus process.