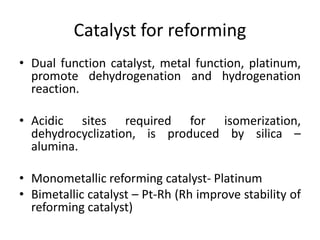
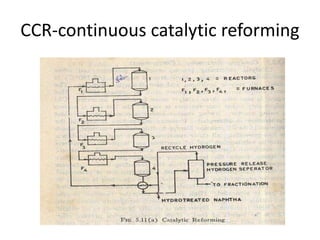
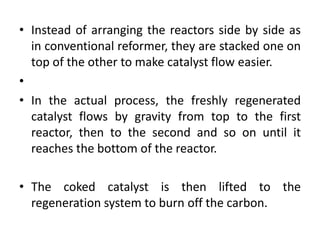
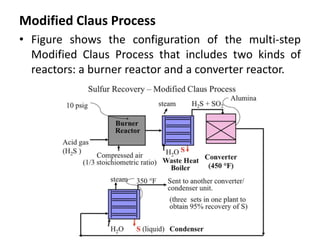
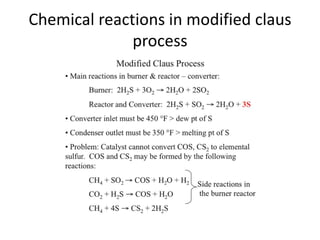
Catalytic reforming is a process that raises the octane number of gasoline through converting non-aromatic components to aromatics over a catalyst. This is done using a platinum or platinum-rhodium catalyst with silica-alumina support in the presence of hydrogen. Bimetallic catalysts provide benefits like higher liquid yields, octane and hydrogen production while allowing lower pressures and hydrogen usage. Continuous catalytic reforming improves on conventional reforming by having catalyst flow continuously through stacked reactors from top to bottom with spent catalyst regenerating at the bottom. The modified Claus process recovers sulfur from hydrogen sulfide by partially oxidizing it to sulfur dioxide in a burner, then catalytically converting the sulfur compounds to elemental