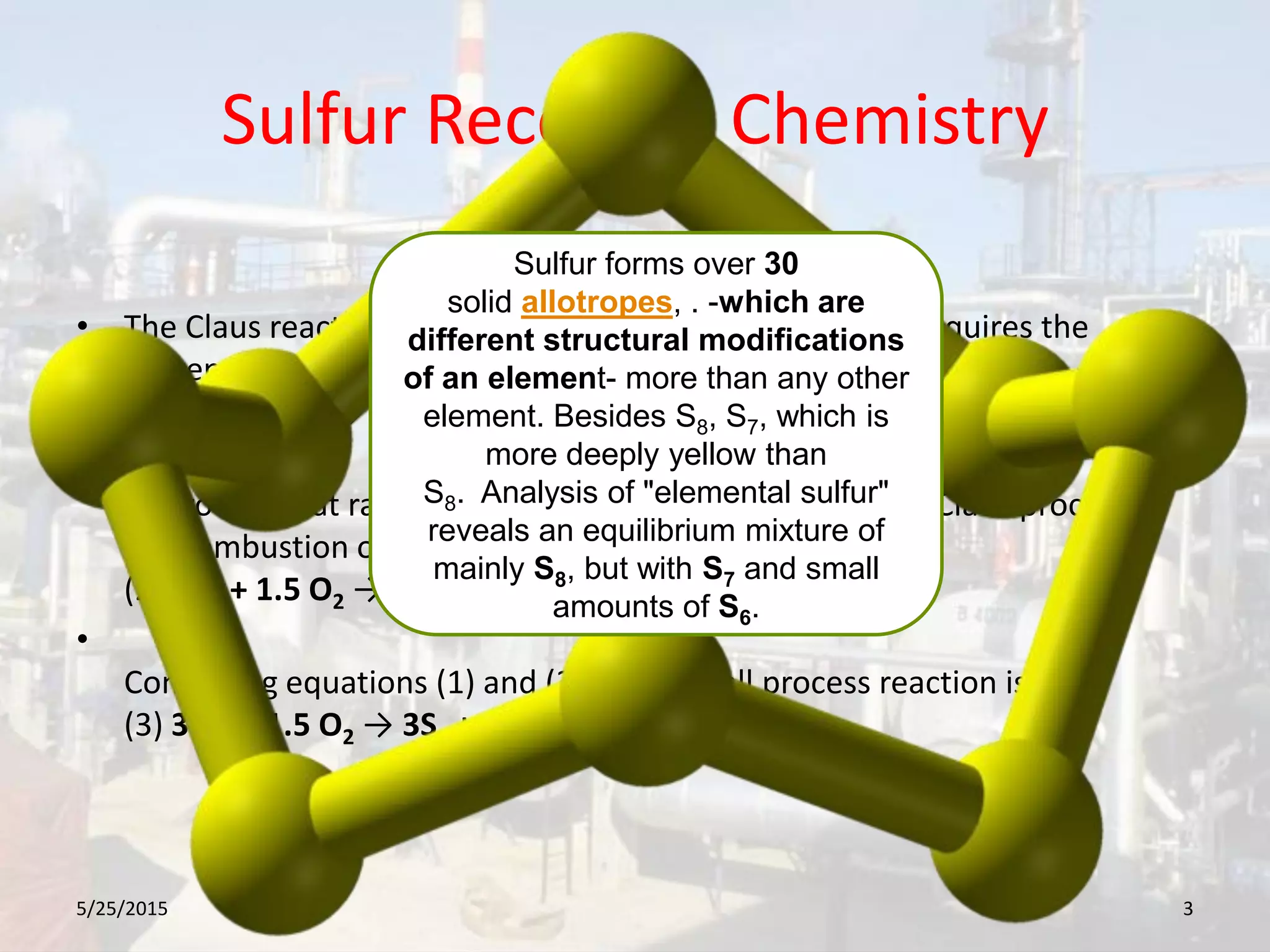
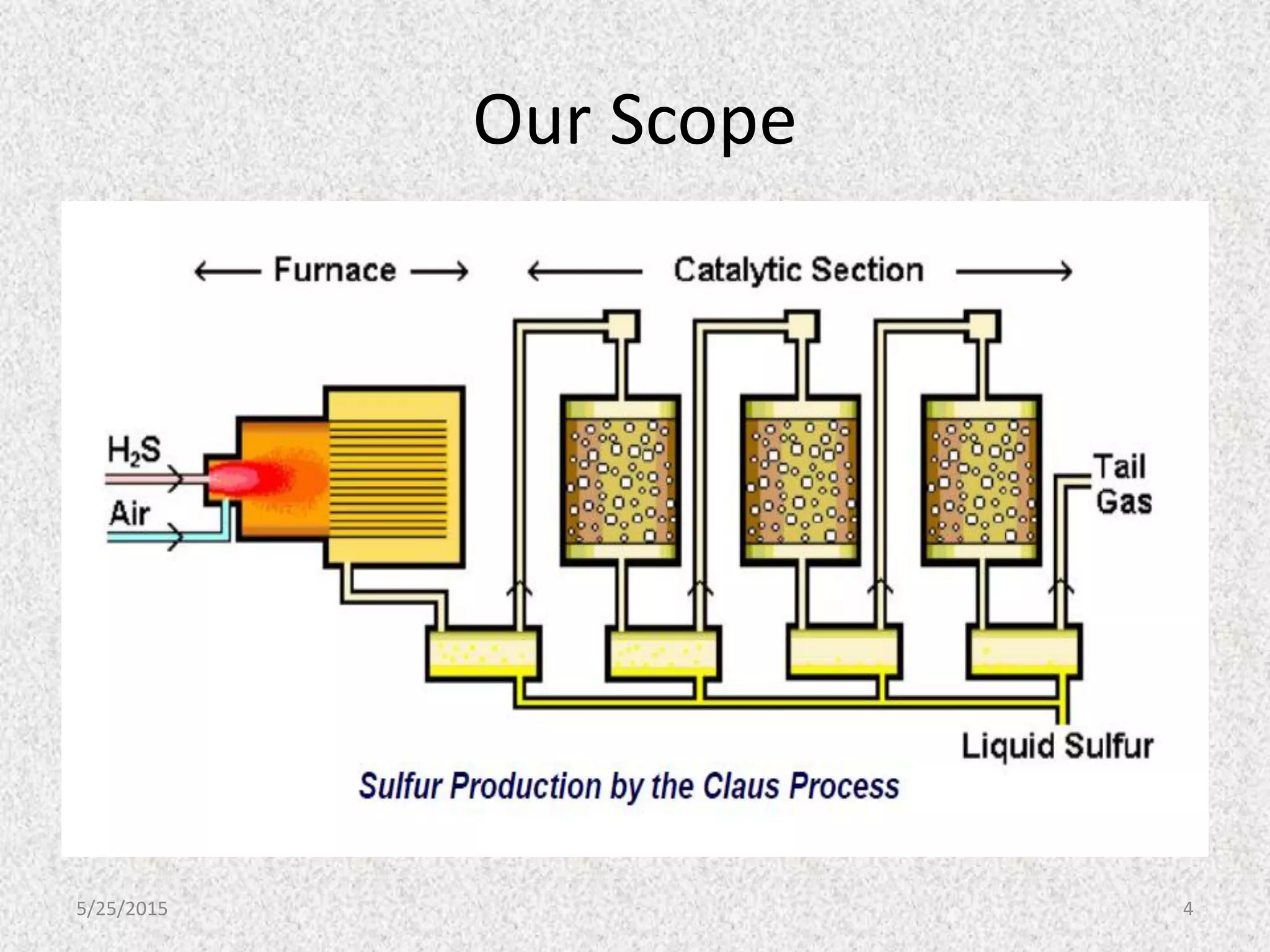
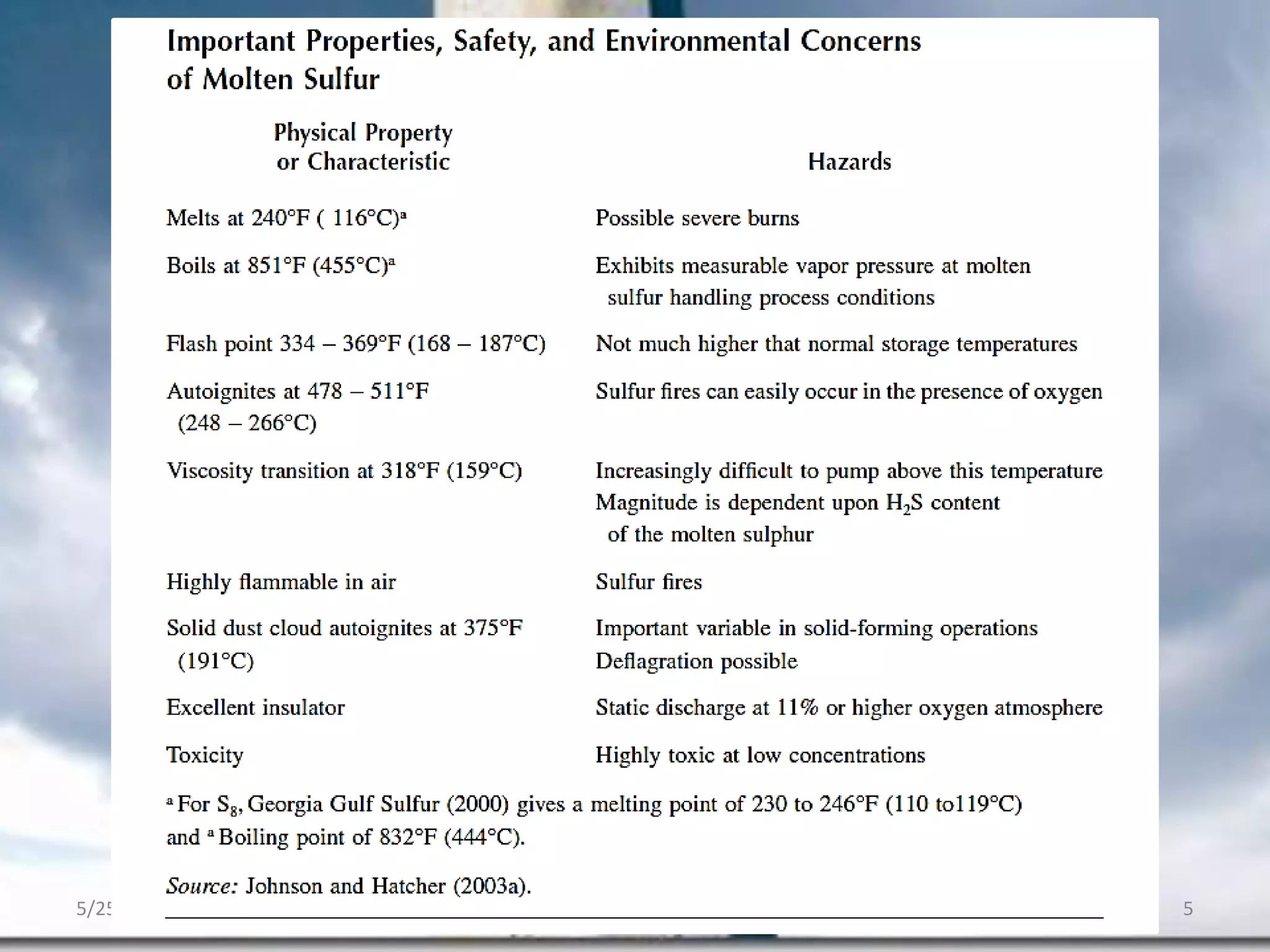
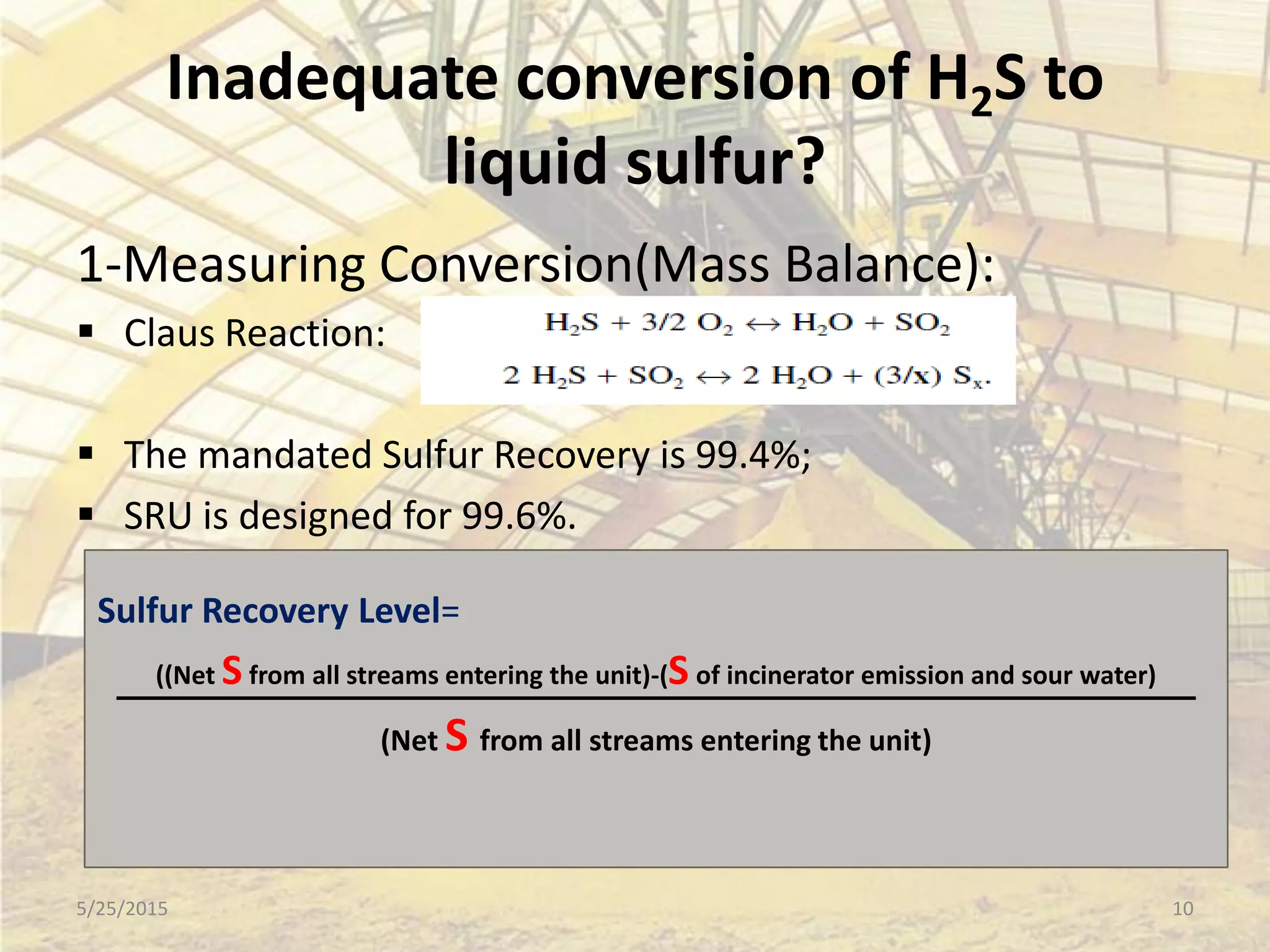
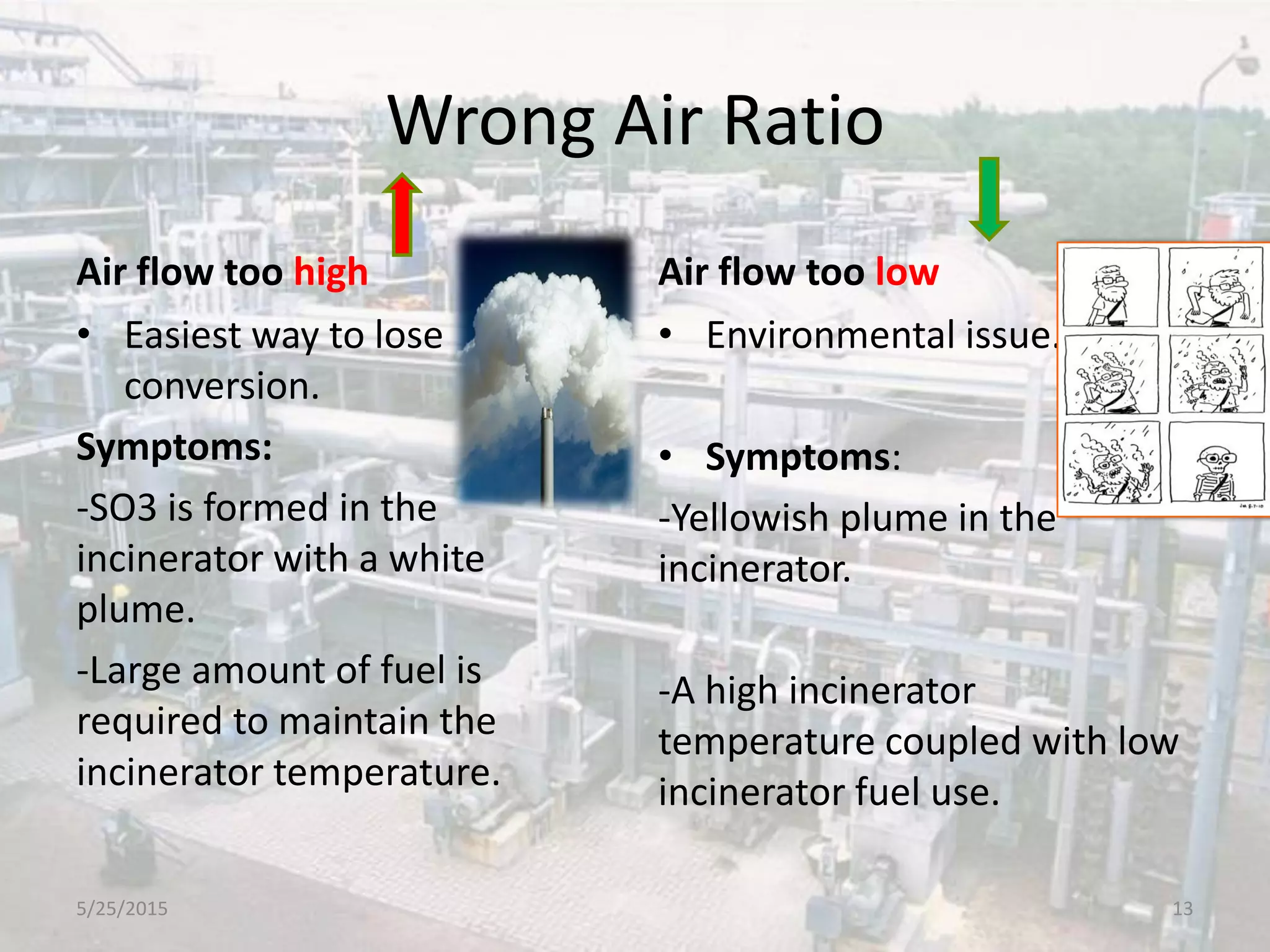
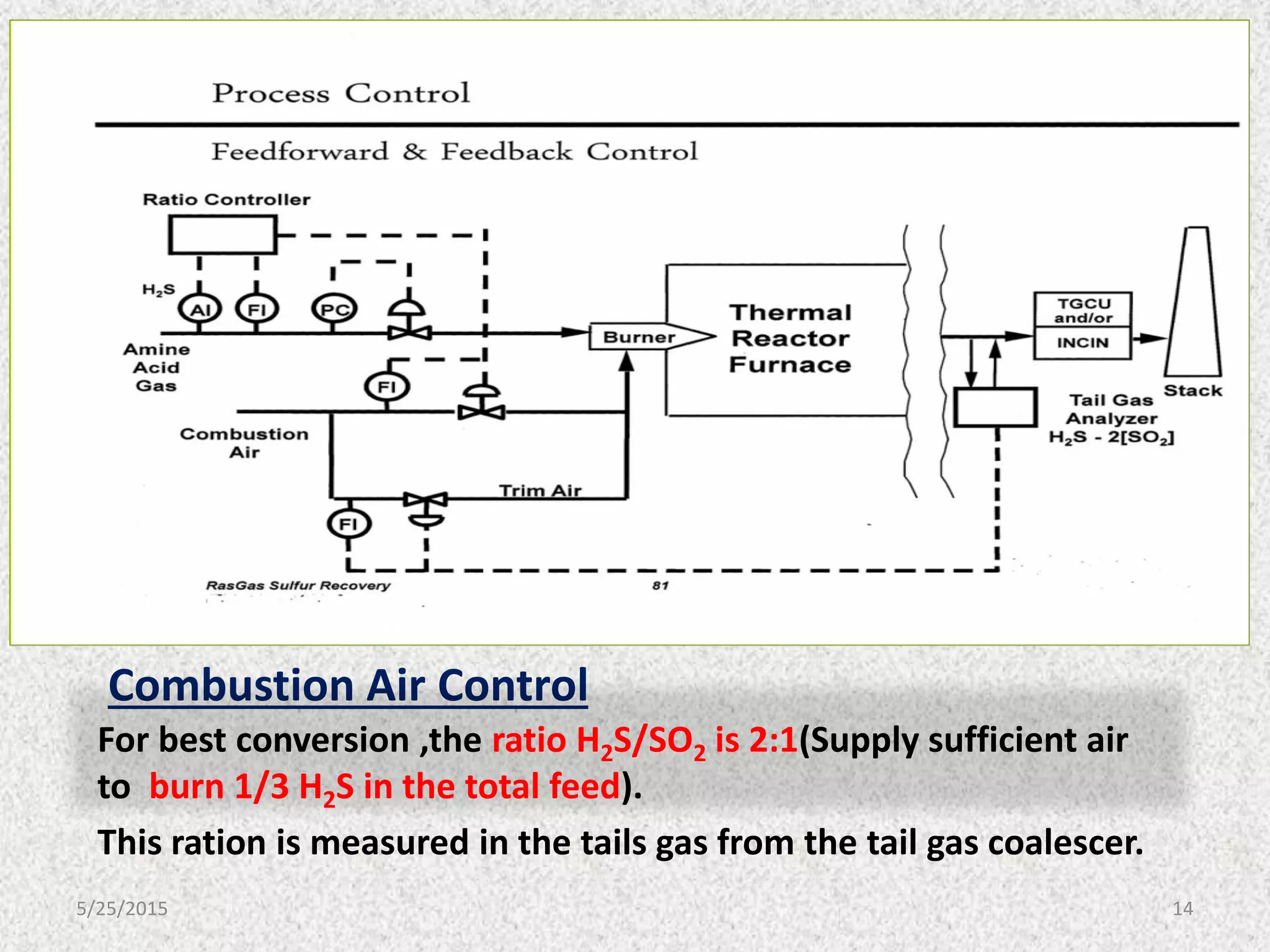
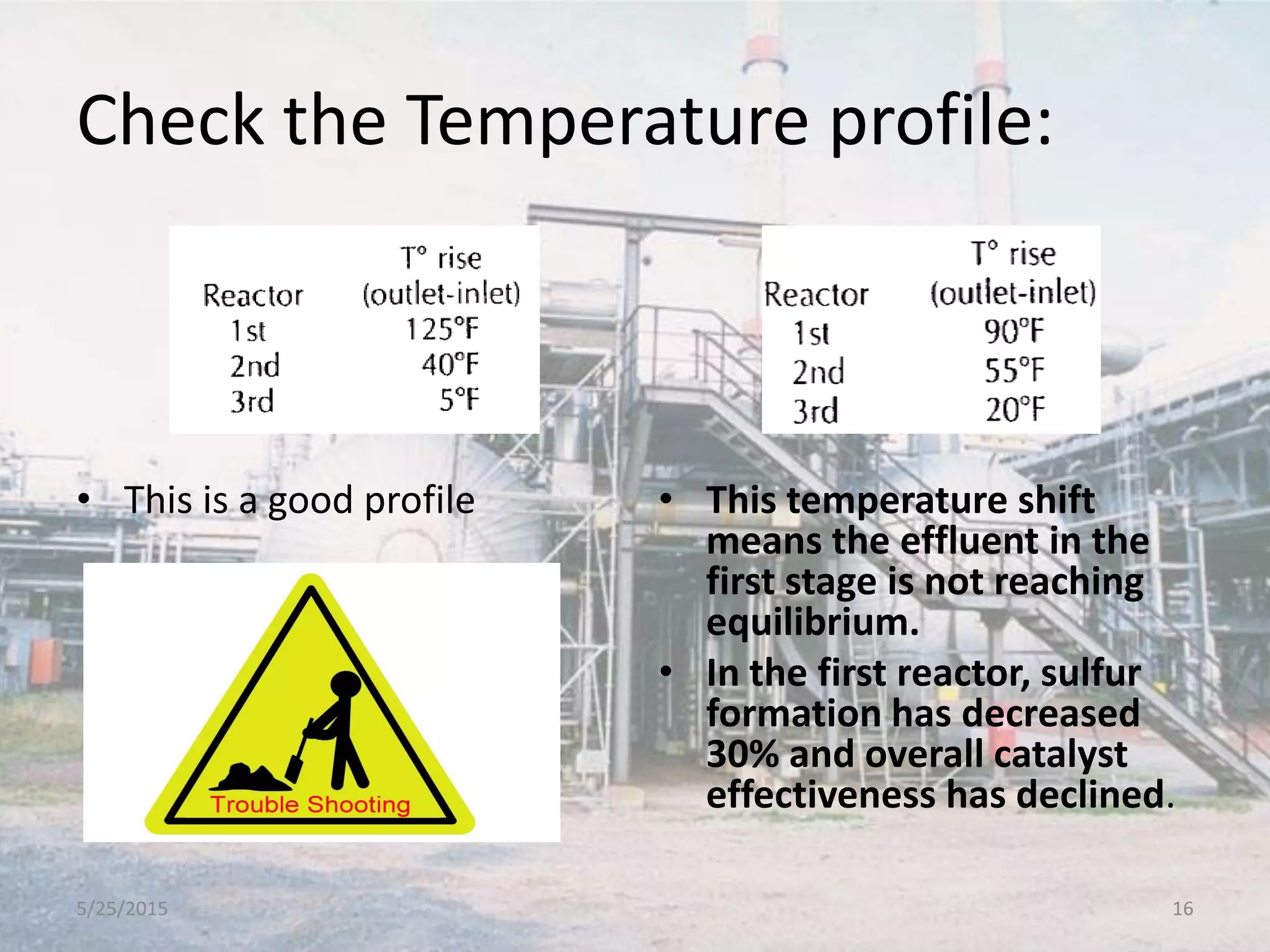
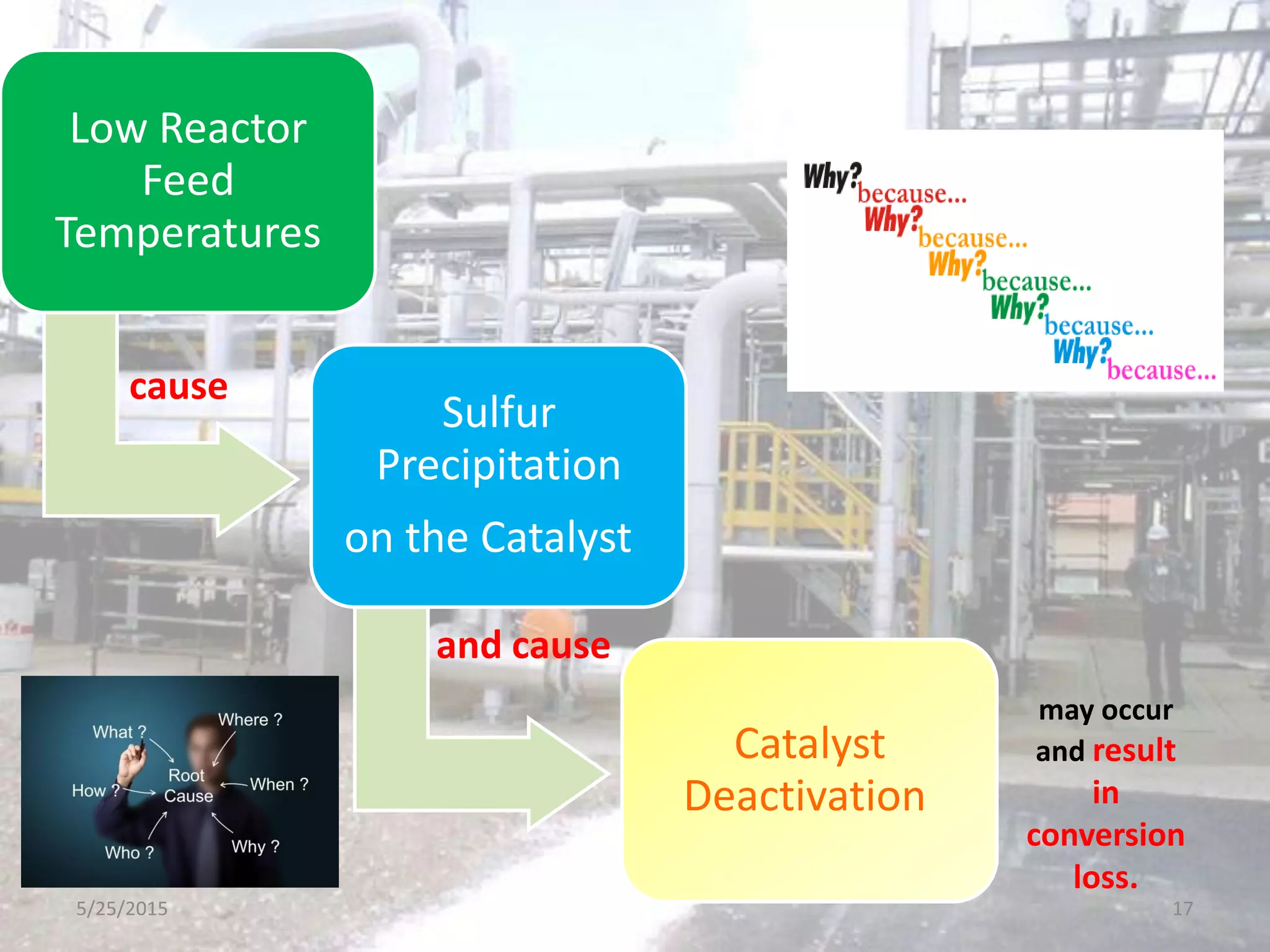
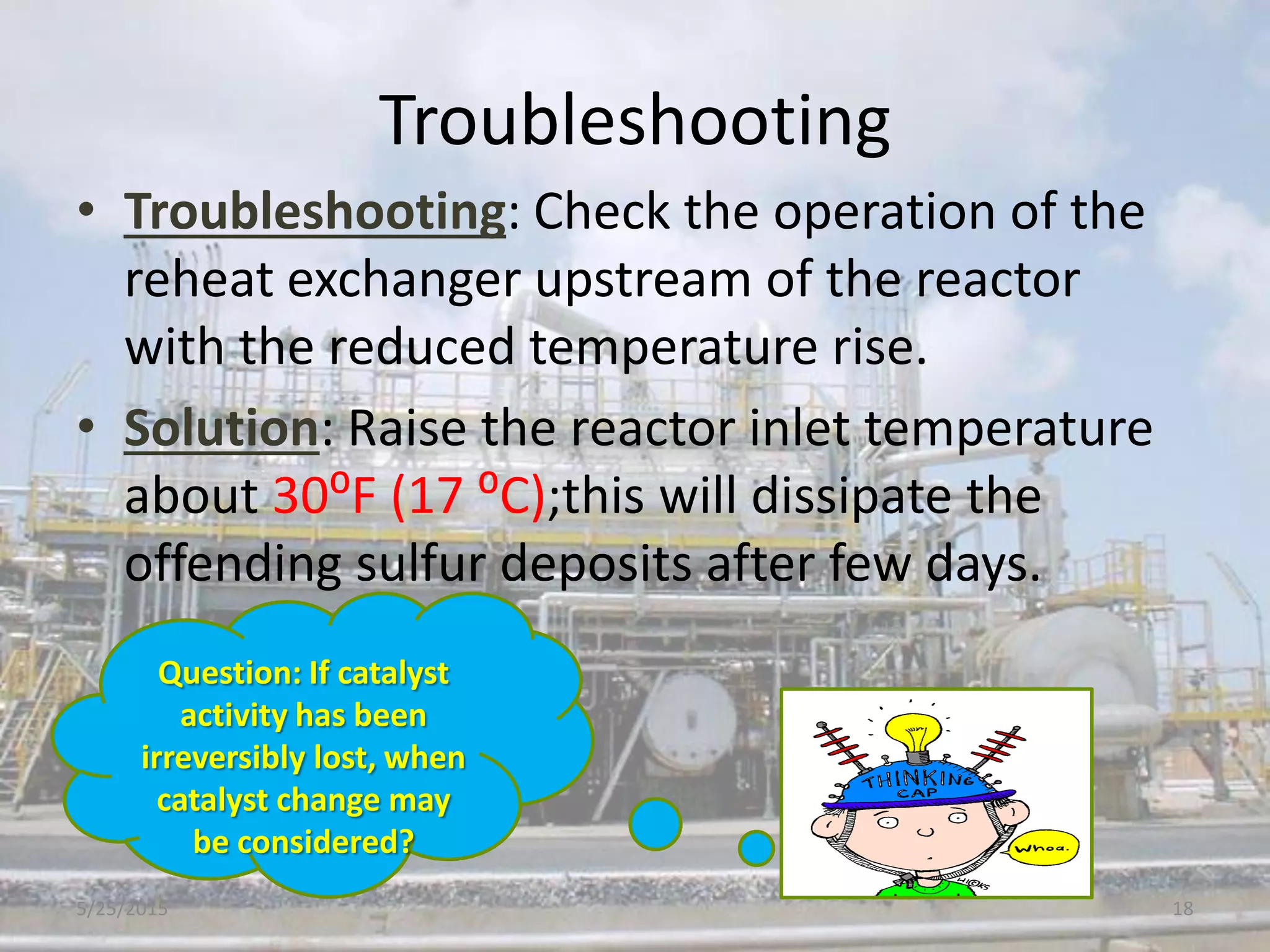
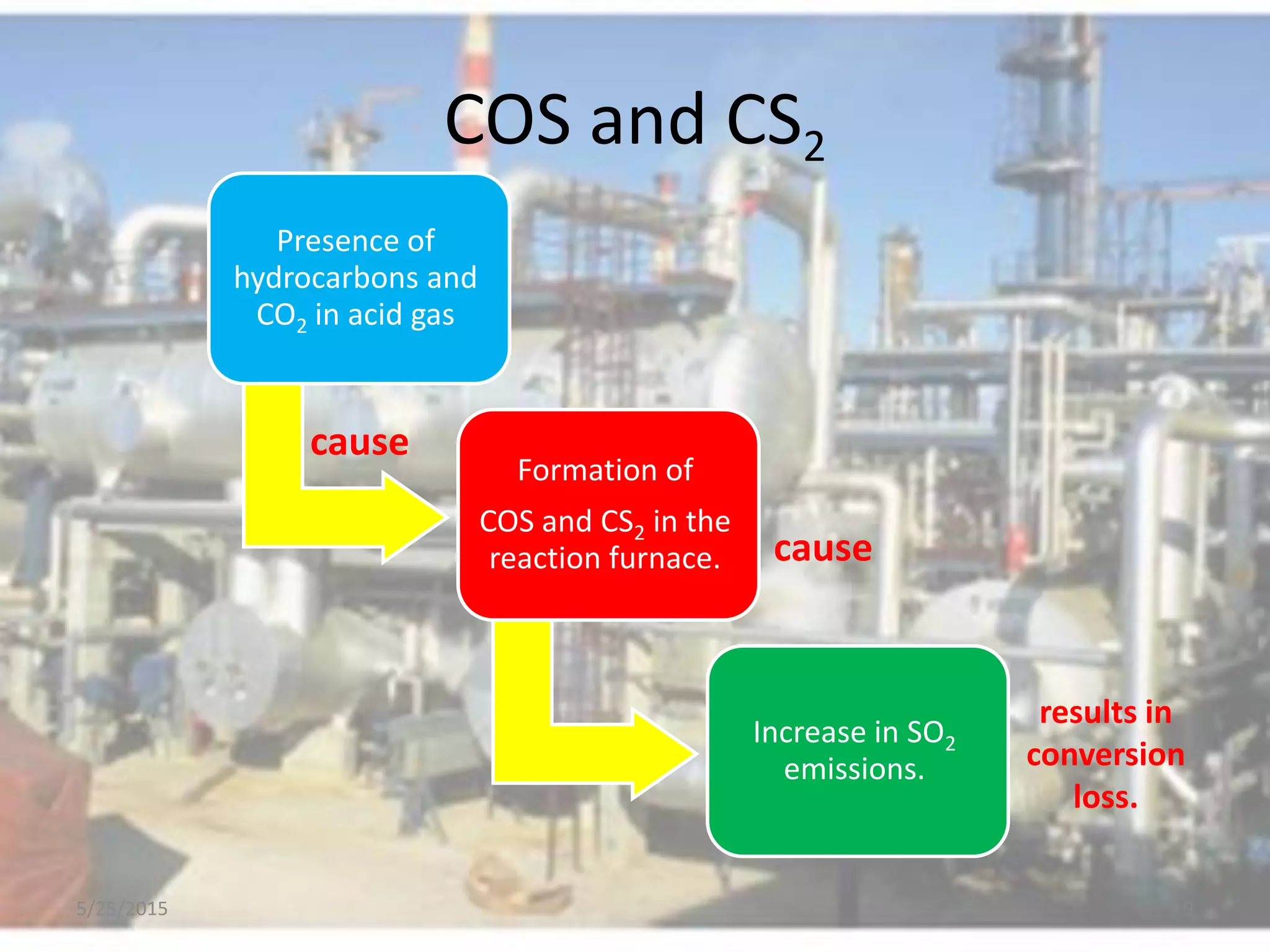
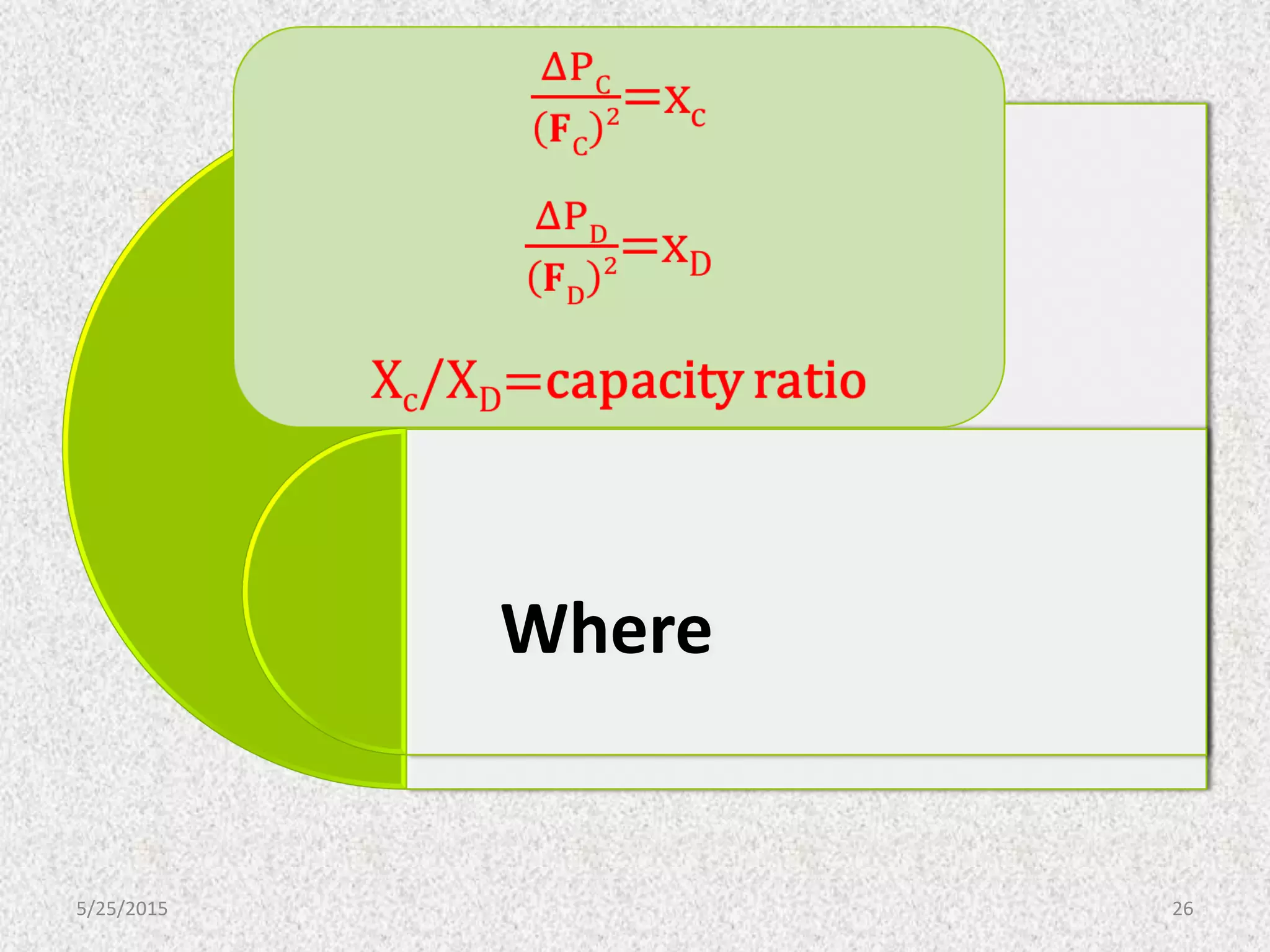
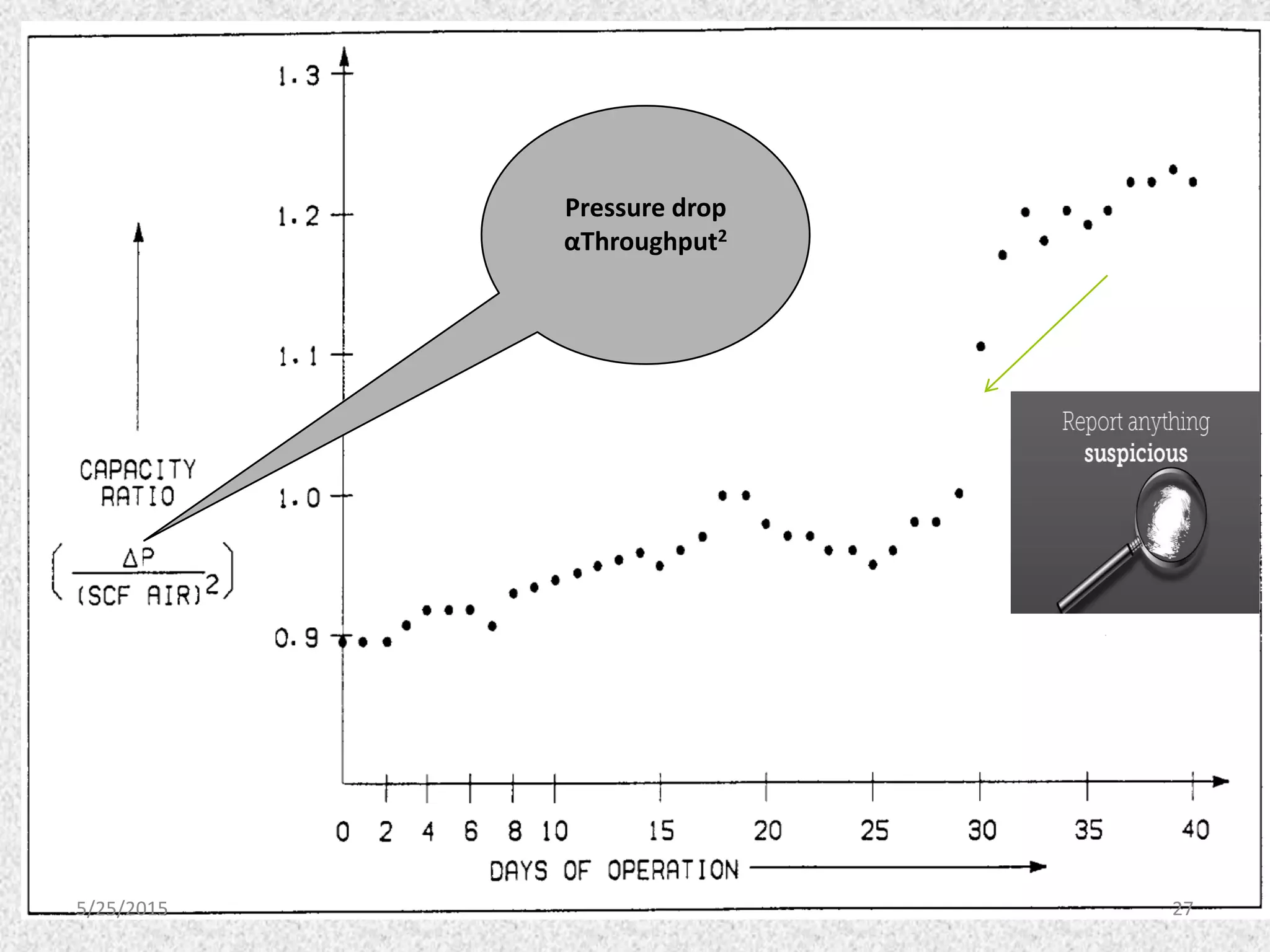
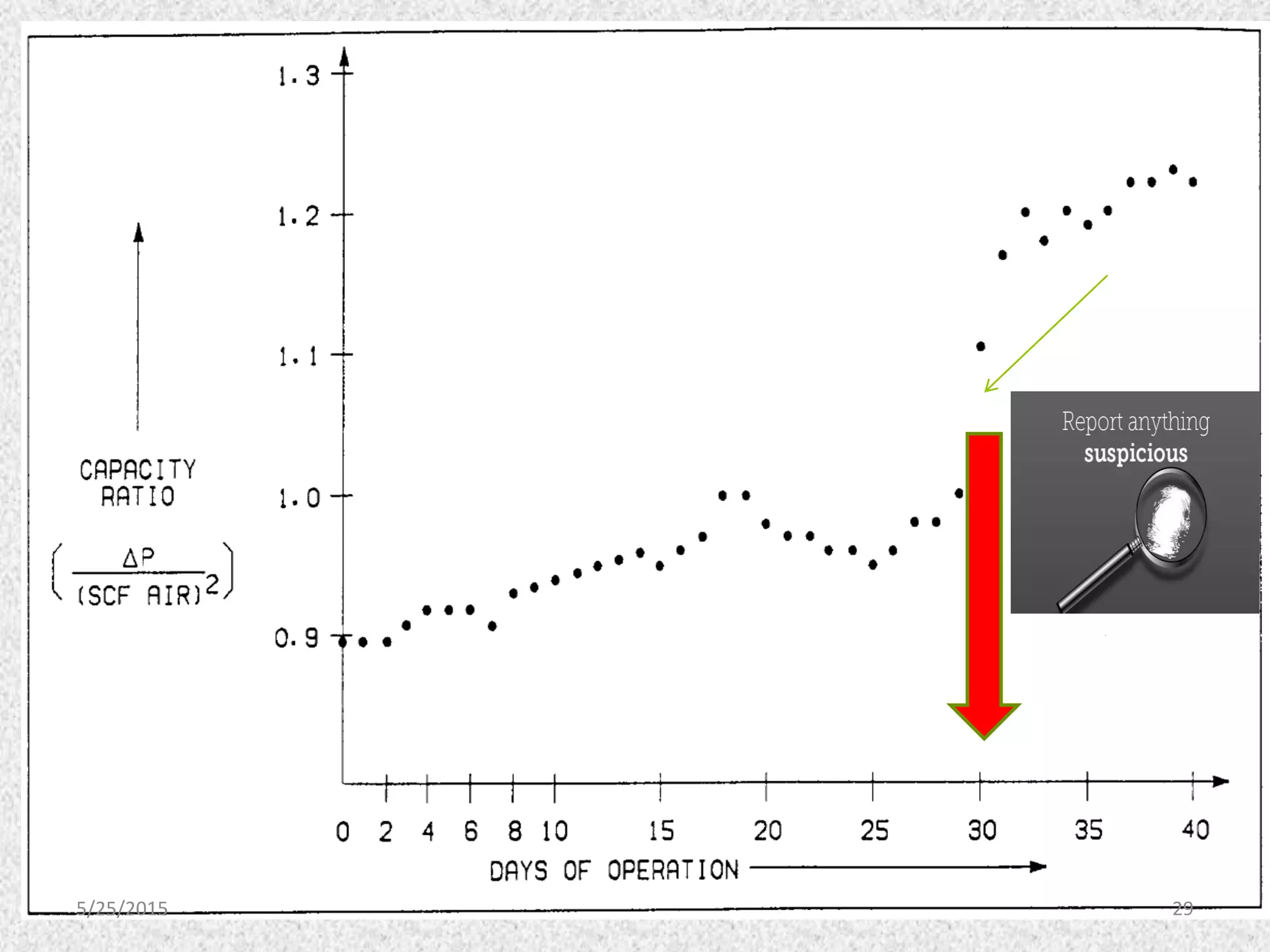
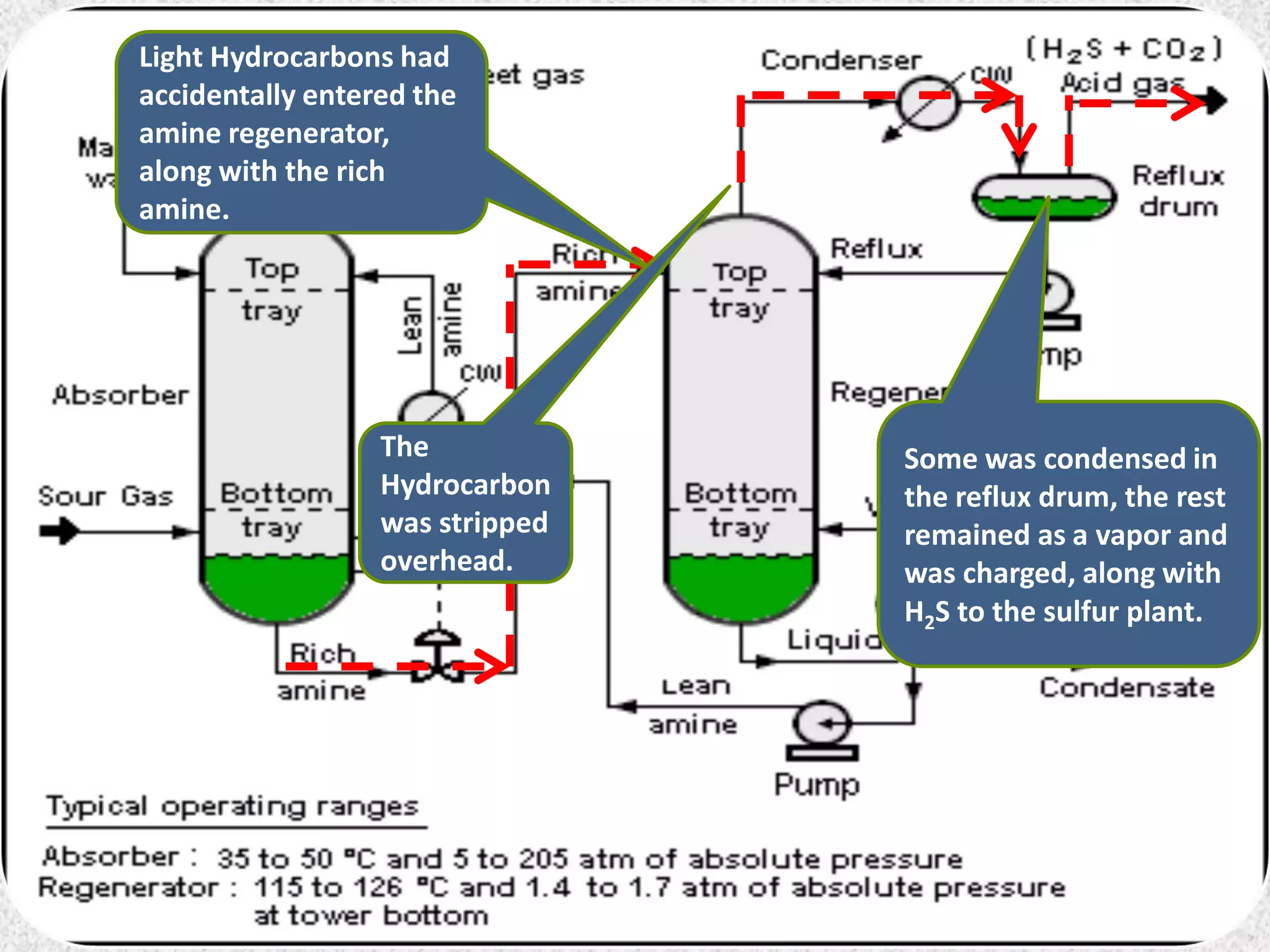
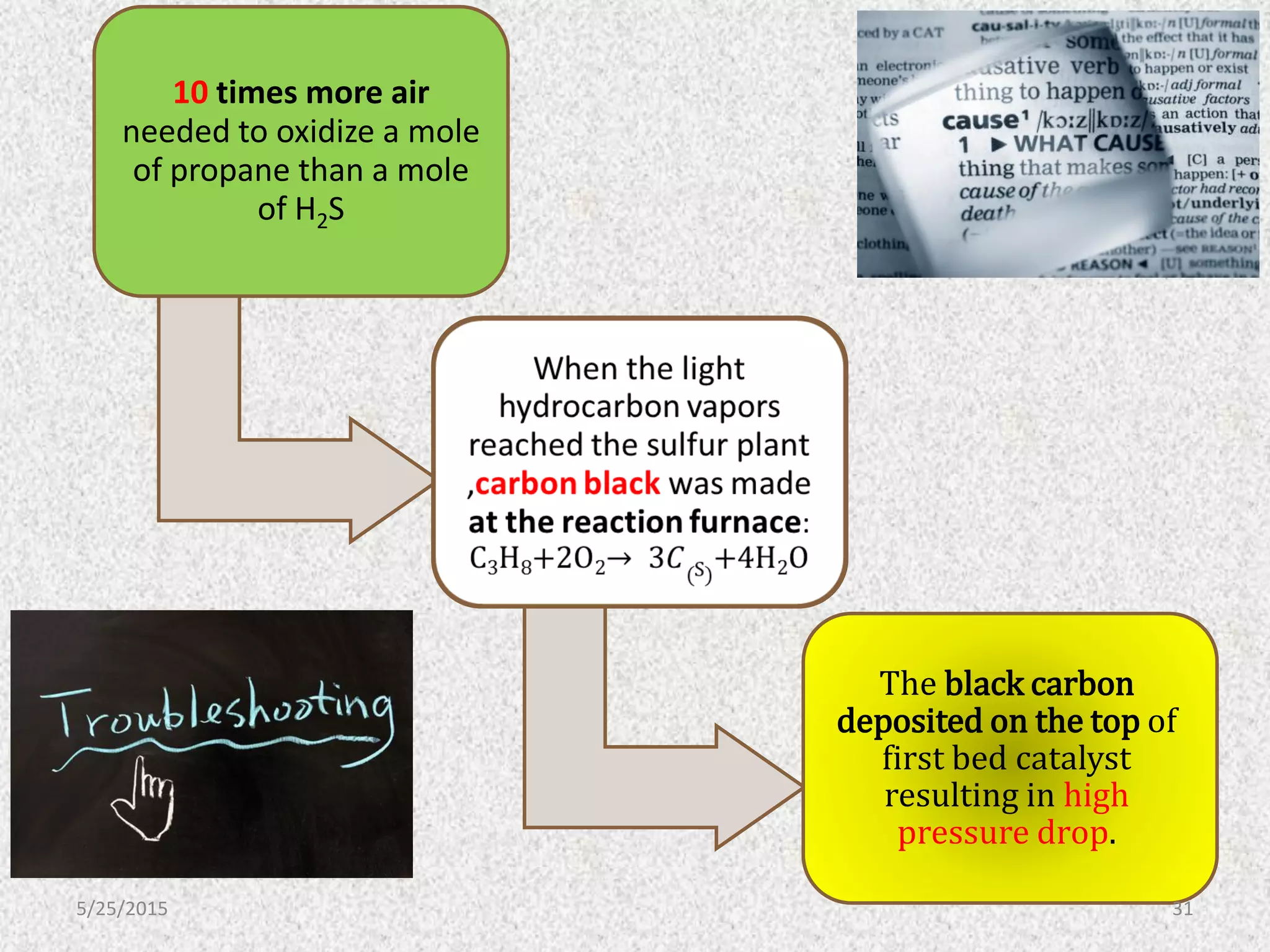
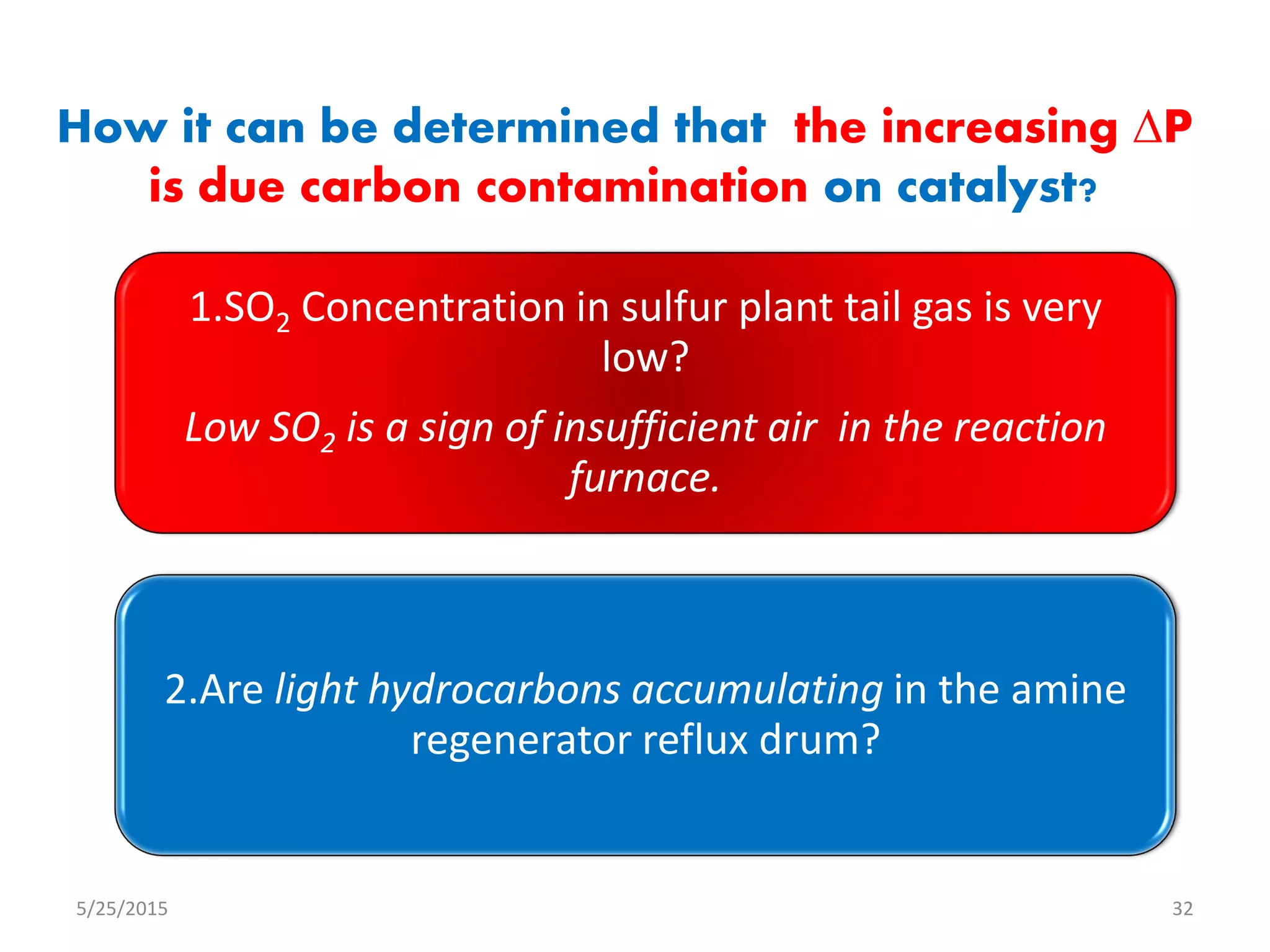
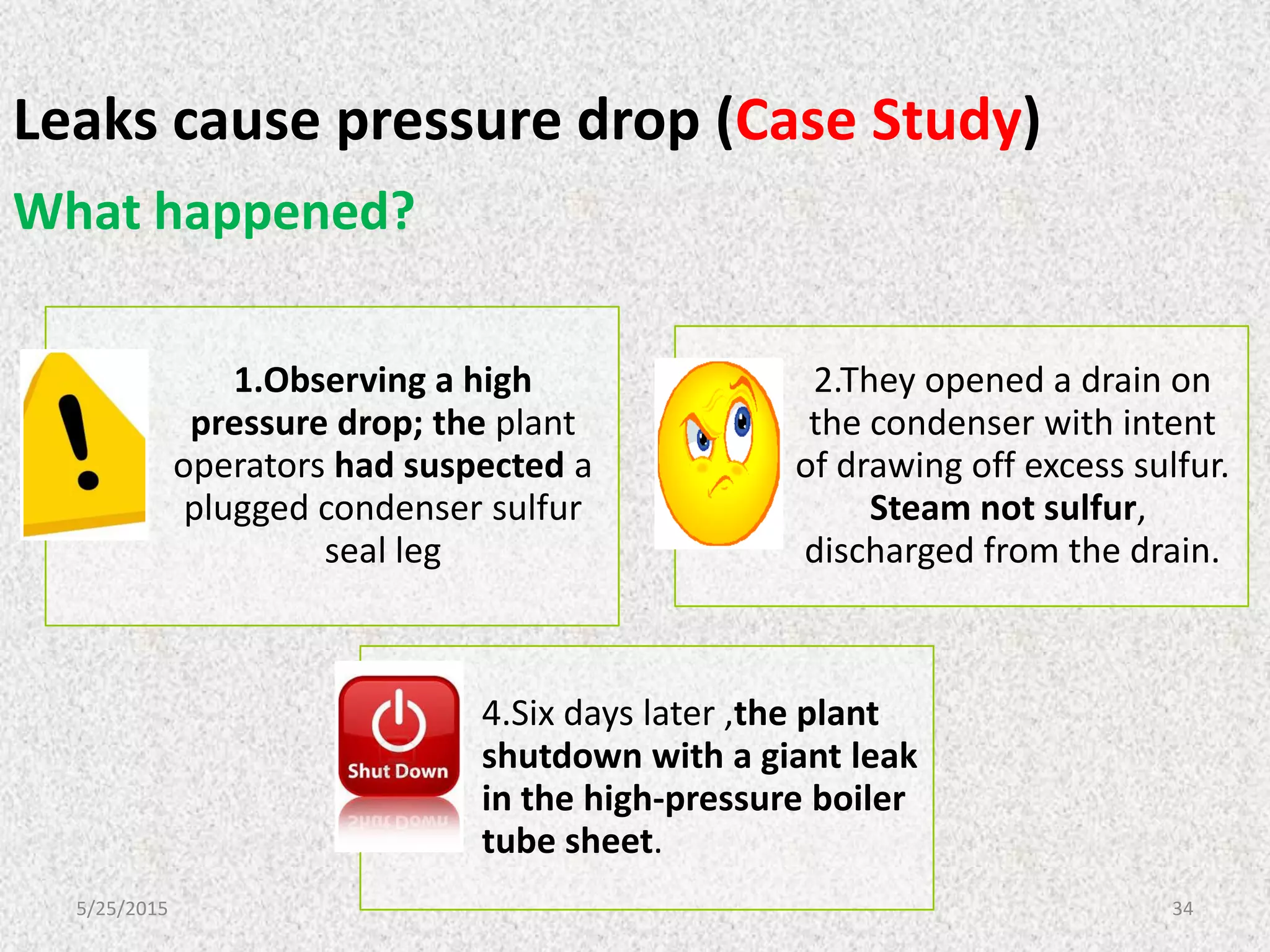
This document discusses troubleshooting issues that can occur in sulfur recovery units (SRUs). It begins with an overview of sulfur chemistry and the Claus process for converting hydrogen sulfide to elemental sulfur. Common problems that can cause conversion losses or pressure drops are then examined, such as carbon deposits, leaks, catalyst deactivation, and improper air-to-acid gas ratios. Specific case studies are presented on troubleshooting carbon deposits from hydrocarbon contamination and identifying leaks from declining steam production and rising pressure drops. The document emphasizes the importance of continuous monitoring and preventative maintenance to address problems in SRUs before catastrophic failures occur.