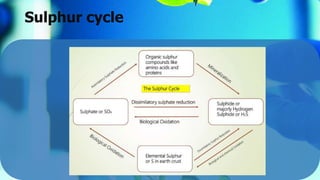
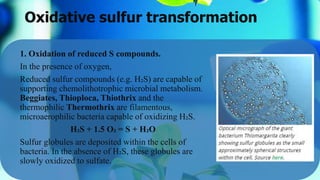
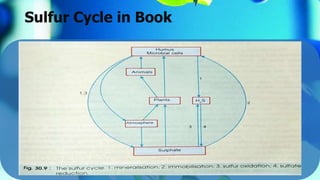
The document outlines the sulfur cycle, detailing oxidative sulfur transformations in five stages, including the oxidation of reduced sulfur compounds and the use of nitrate as electron acceptors. It describes the role of specific bacteria such as Beggiatoa and Thiobacillus species in these processes and their impact on soil health and nutrient mobilization. Additionally, the document highlights the phototrophic oxidation of hydrogen sulfide by photosynthetic sulfur bacteria.