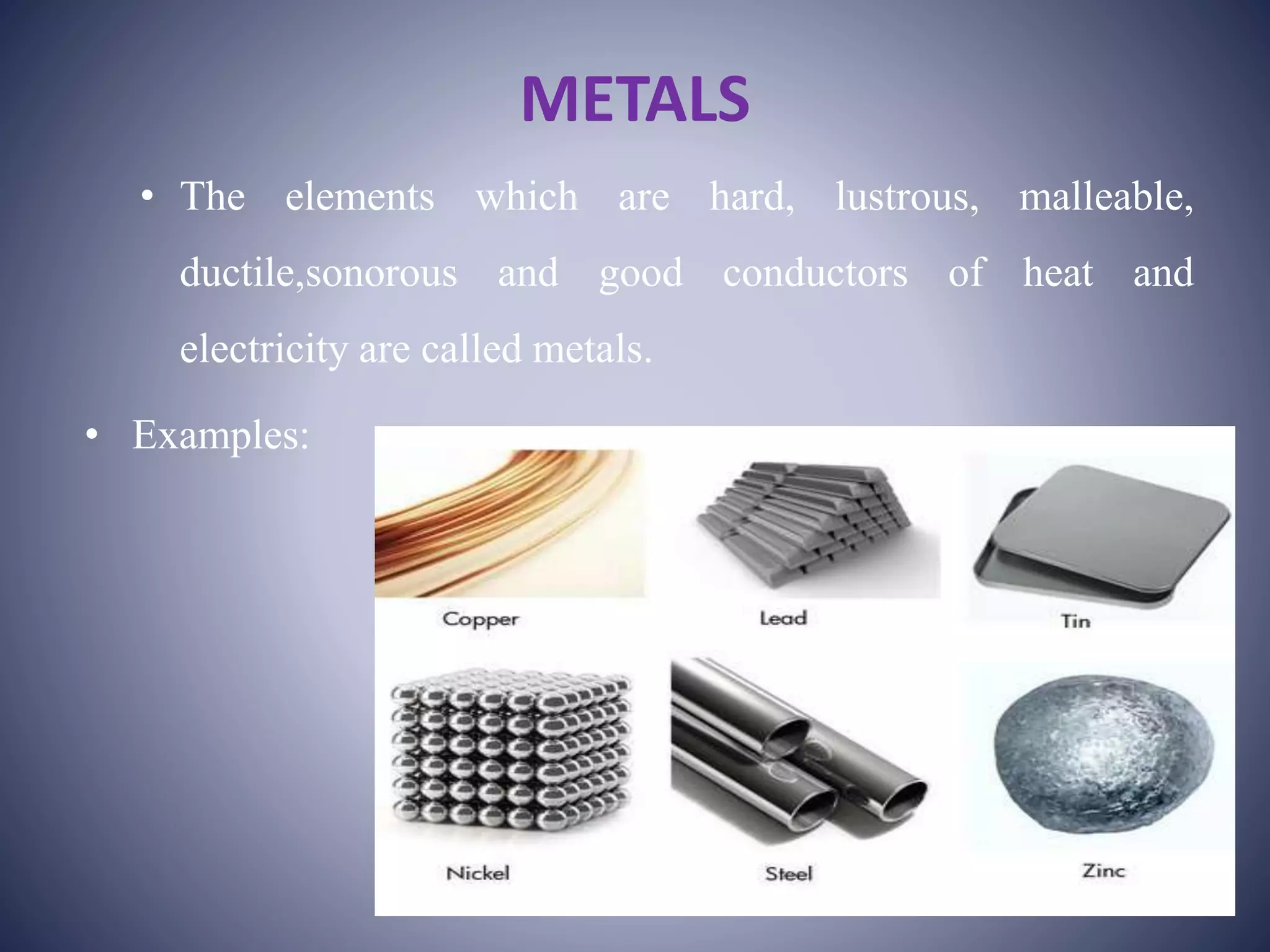
This document discusses the classification of elements into metals, non-metals, metalloids, and noble gases. Metals are hard, lustrous, malleable, ductile, sonorous, and good conductors of heat and electricity. Non-metals generally do not shine, are soft, are non-conductors of heat and electricity, and do not have properties of malleability or ductility. Metalloids possess some properties of both metals and non-metals, having conductivity between the two and being solid, ductile, and brittle semi-conductors. Noble gases are colorless, odorless, monoatomic gases that are liquifiable at low temperatures and have low melting and boiling points