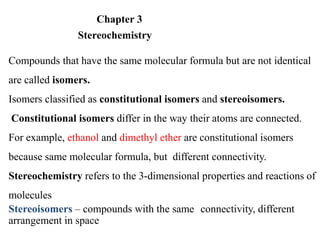
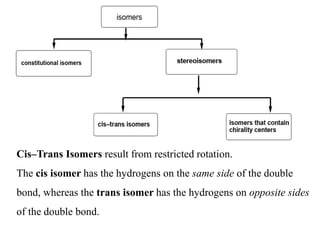
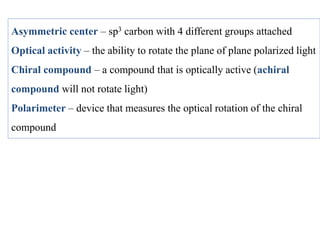
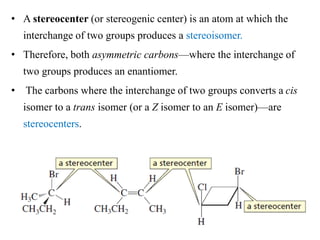
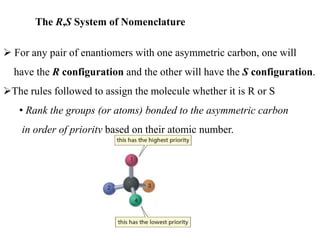
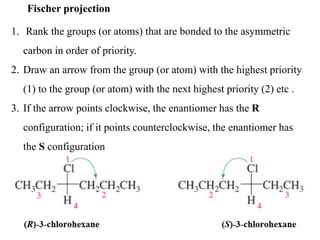
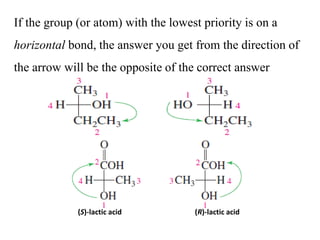
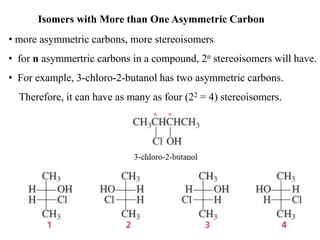
This document discusses stereochemistry and isomers. It defines constitutional and stereoisomers. Stereoisomers include cis-trans isomers which result from restricted bond rotation, and enantiomers which are non-superimposable mirror images. Chiral compounds contain an asymmetric carbon bonded to four different groups and can rotate polarized light. The R/S system is used to designate stereochemistry at chiral centers. Compounds with multiple chiral centers can give rise to diastereomers and meso compounds.