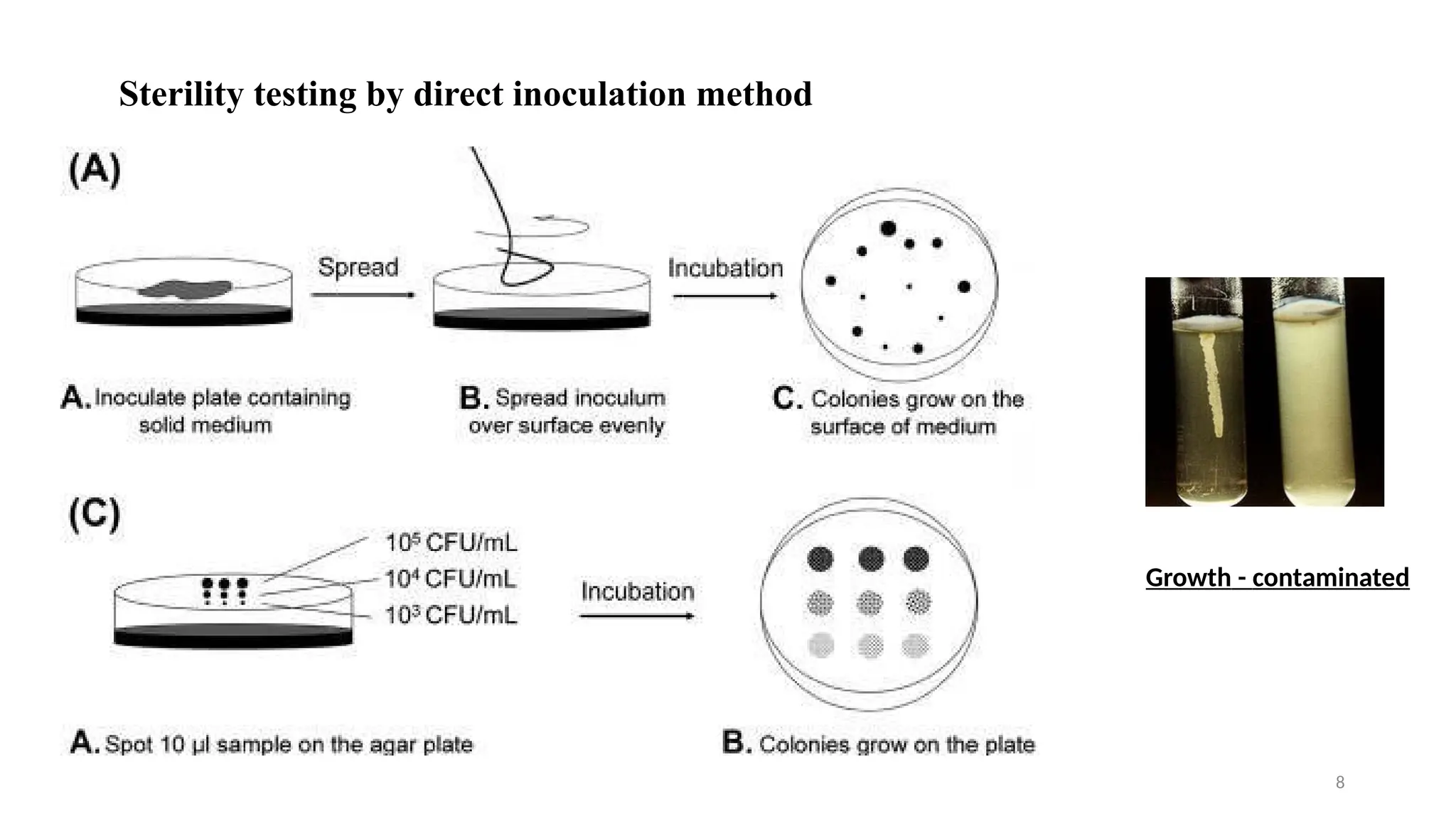
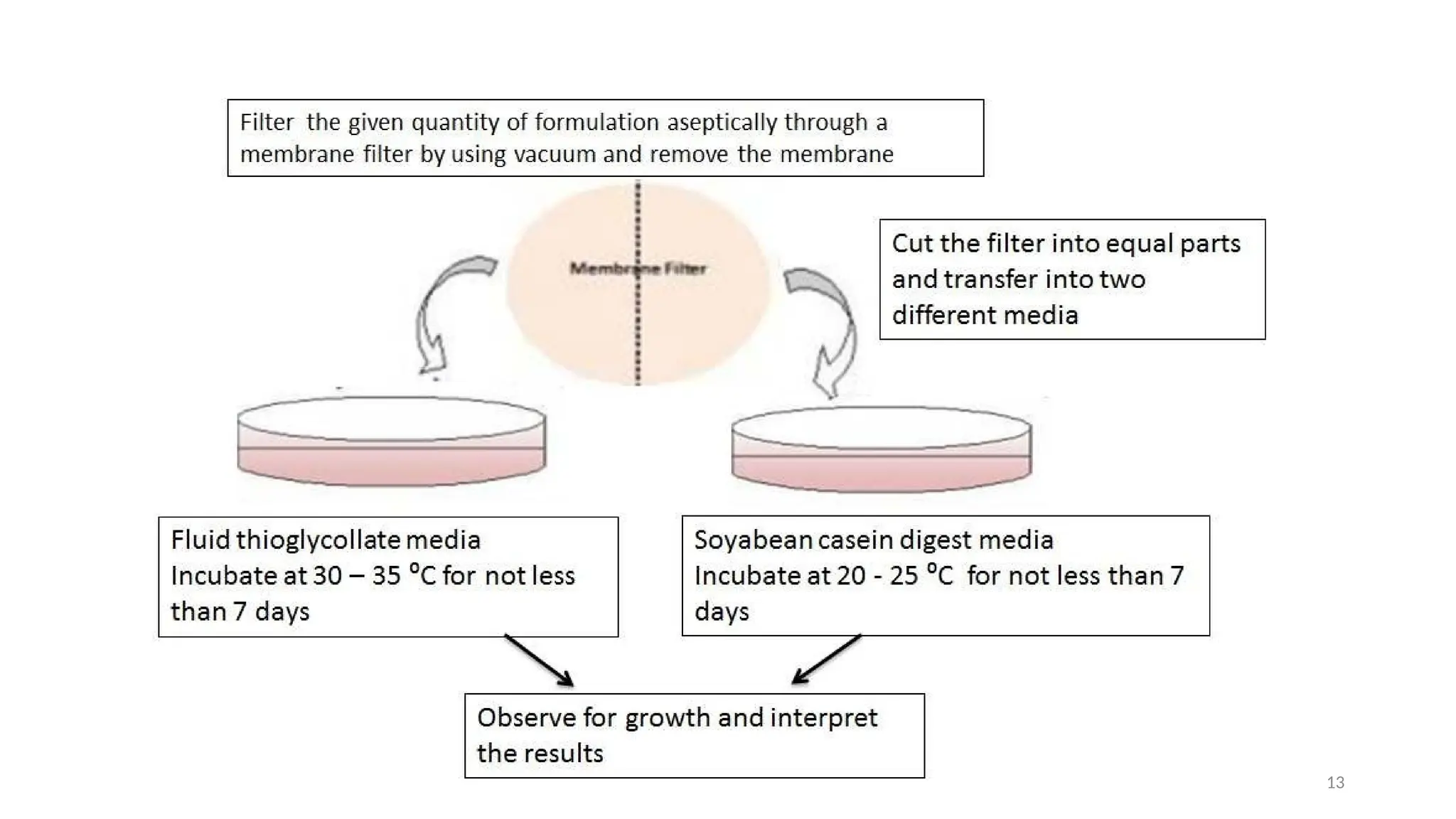
The document outlines the importance and methods of sterility testing for pharmaceutical products, specifically using direct inoculation and membrane filtration techniques. It details the types of culture media utilized, the significance of positive and negative controls, and the procedures for testing various products. The findings determine whether a product complies with sterility standards based on the presence or absence of microbial growth.