Embed presentation
Downloaded 32 times



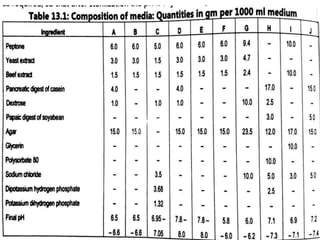

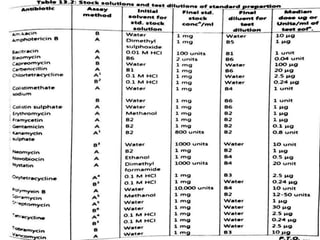

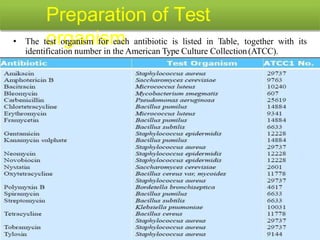



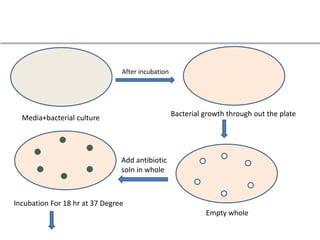
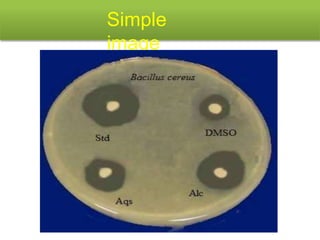
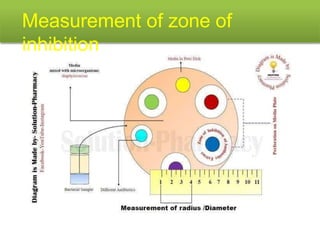
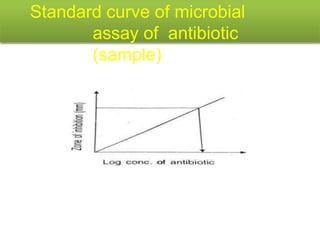
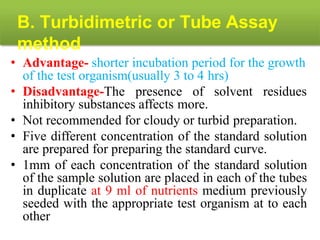
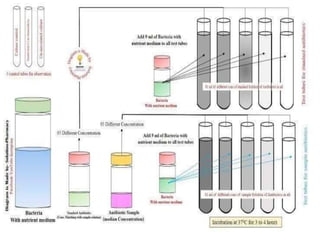

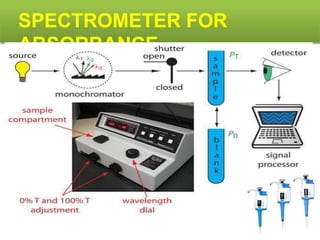
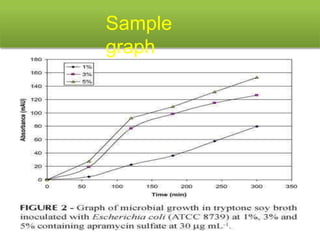
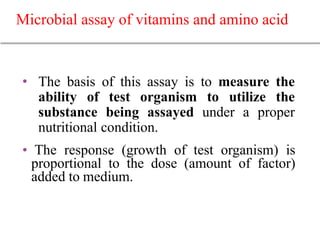

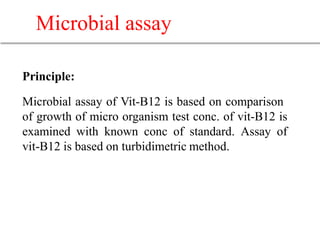

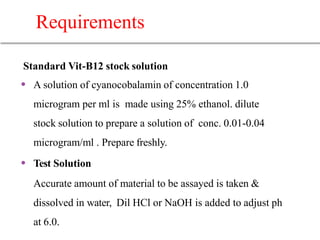
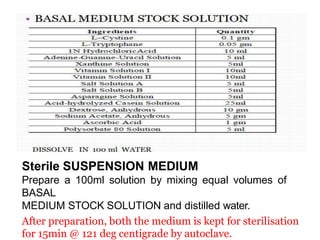
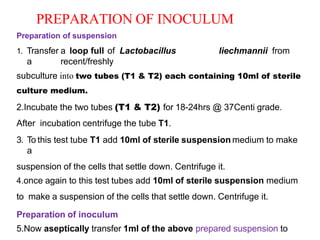

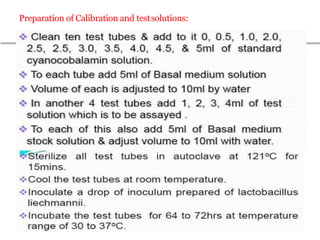
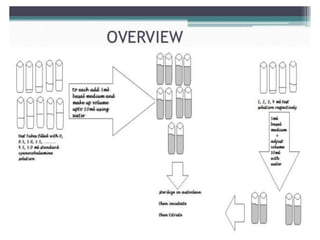

This document describes the process of microbiological assay, specifically as it relates to antibiotics and vitamins. There are two main methods described - the cylinder plate method and the turbidimetric tube assay method. For both methods, standard and test solutions are prepared along with appropriate culture media and test organisms. Zones of inhibition are measured for the cylinder plate method to determine potency, while growth is measured spectrometrically for the turbidimetric tube assay method. Requirements, procedures, and interpretation of results are provided for microbiological assay of both antibiotics and specific vitamins like vitamin B12.

































