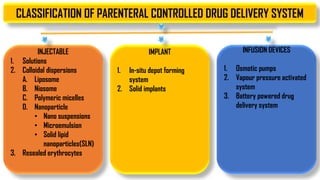
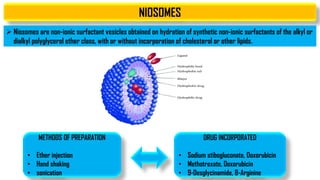
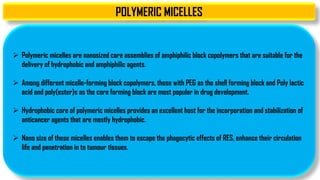
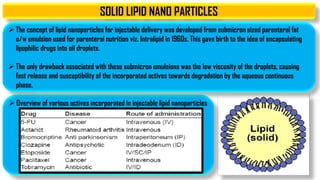
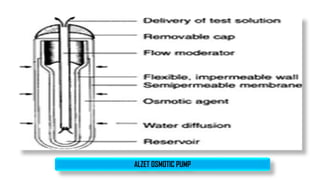
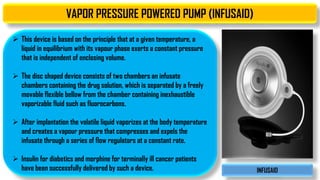
The document discusses parenteral controlled drug delivery systems, which offer effective delivery methods for drugs with poor bioavailability, enhancing patient compliance while reducing the frequency of injections. It covers various technological advancements, including different types of drug delivery systems such as liposomes, niosomes, polymeric micelles, and infusion devices, emphasizing their advantages and applications in targeted and sustained drug release. The document also highlights specific examples like the Atrigel® system and Alzet osmotic pump, demonstrating their capabilities in improving drug delivery efficiencies.