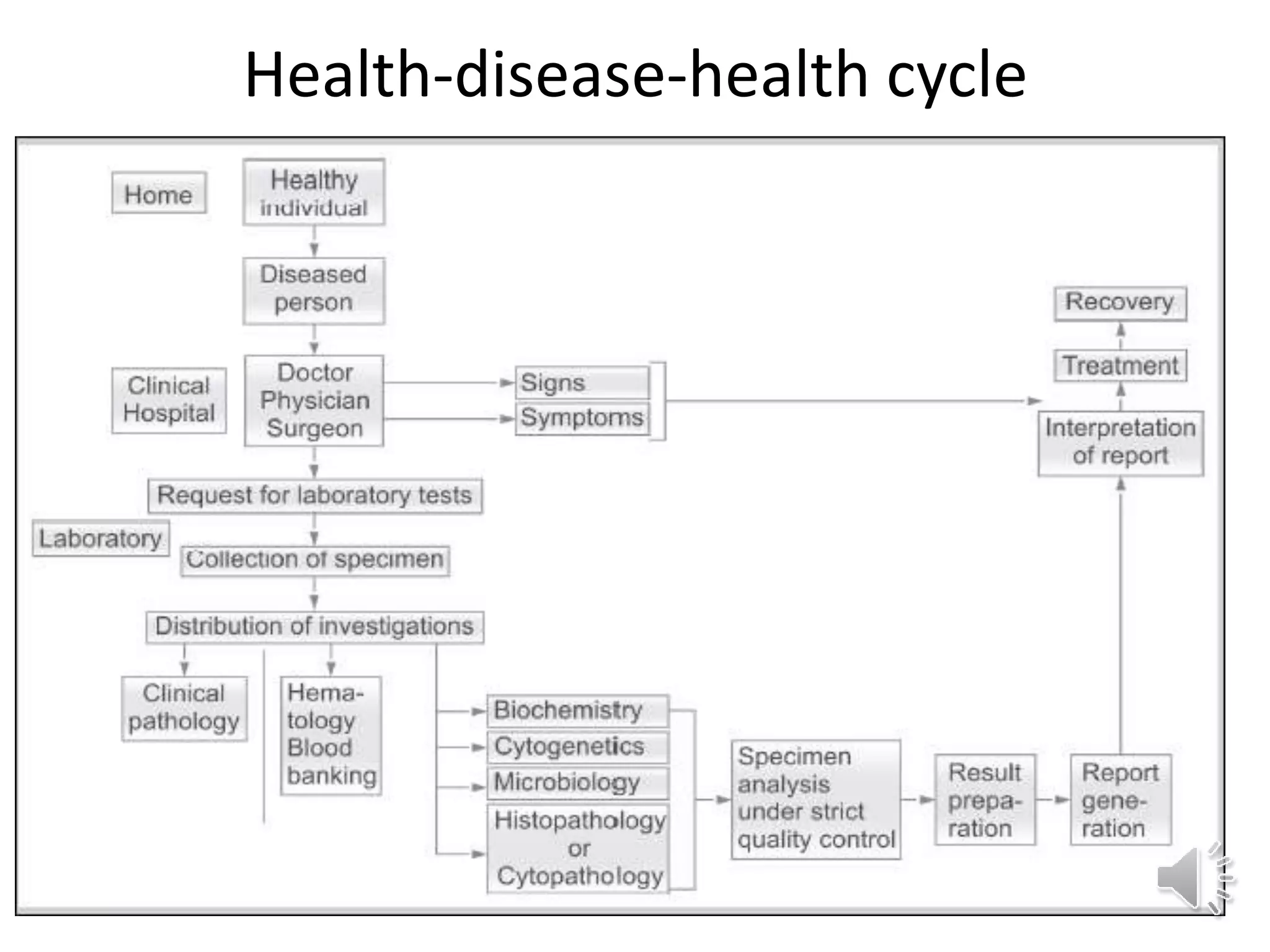
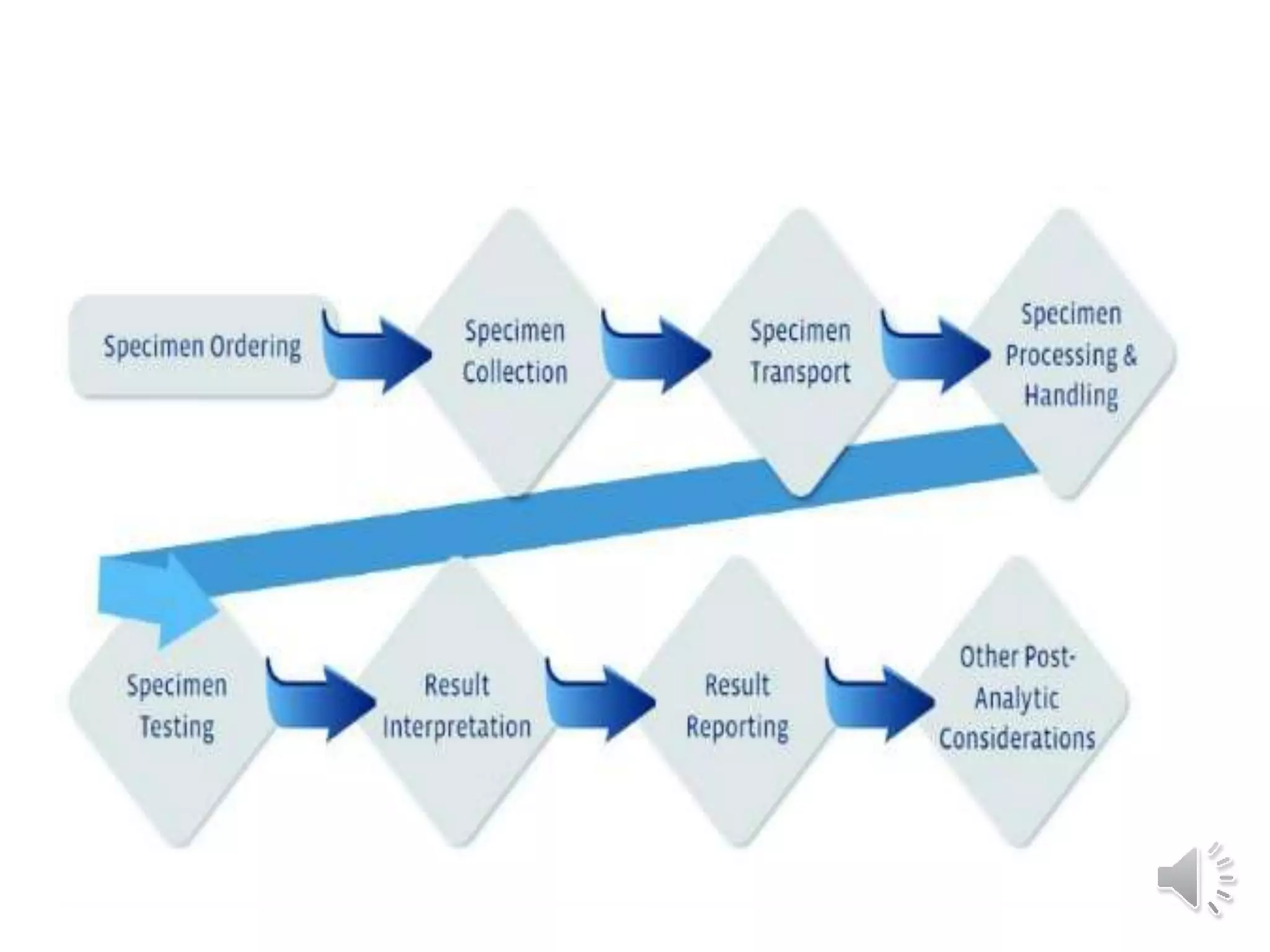
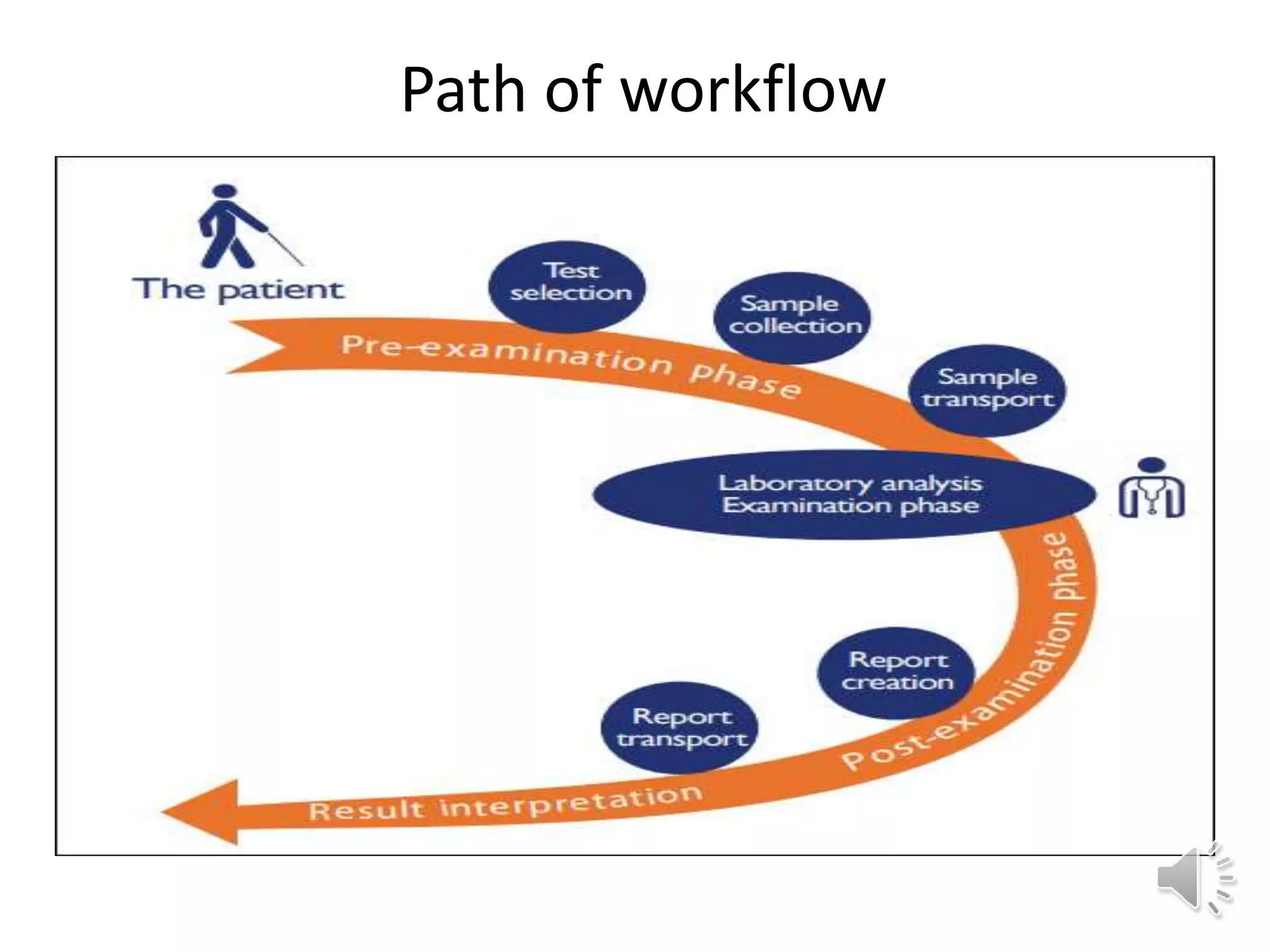
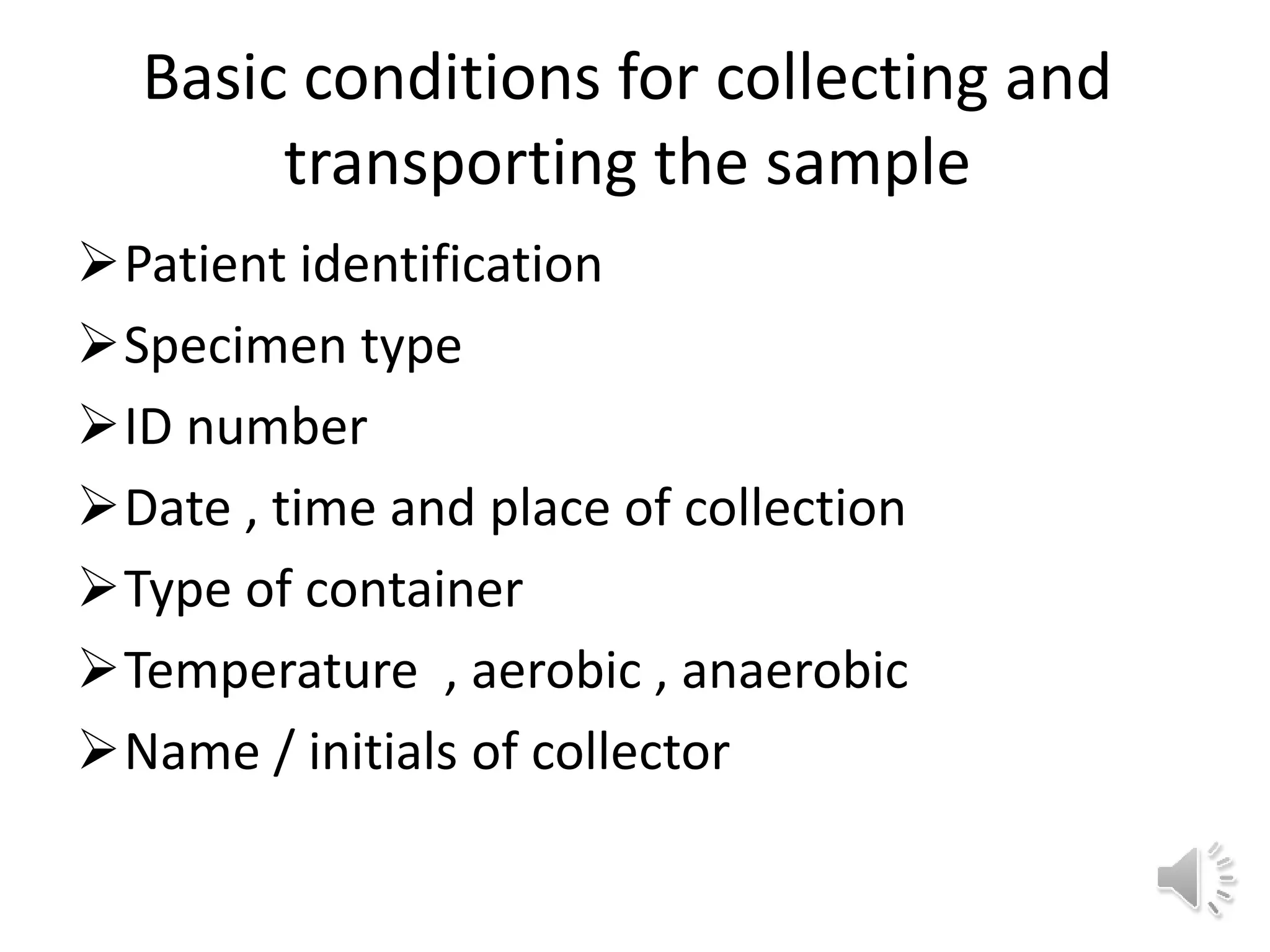
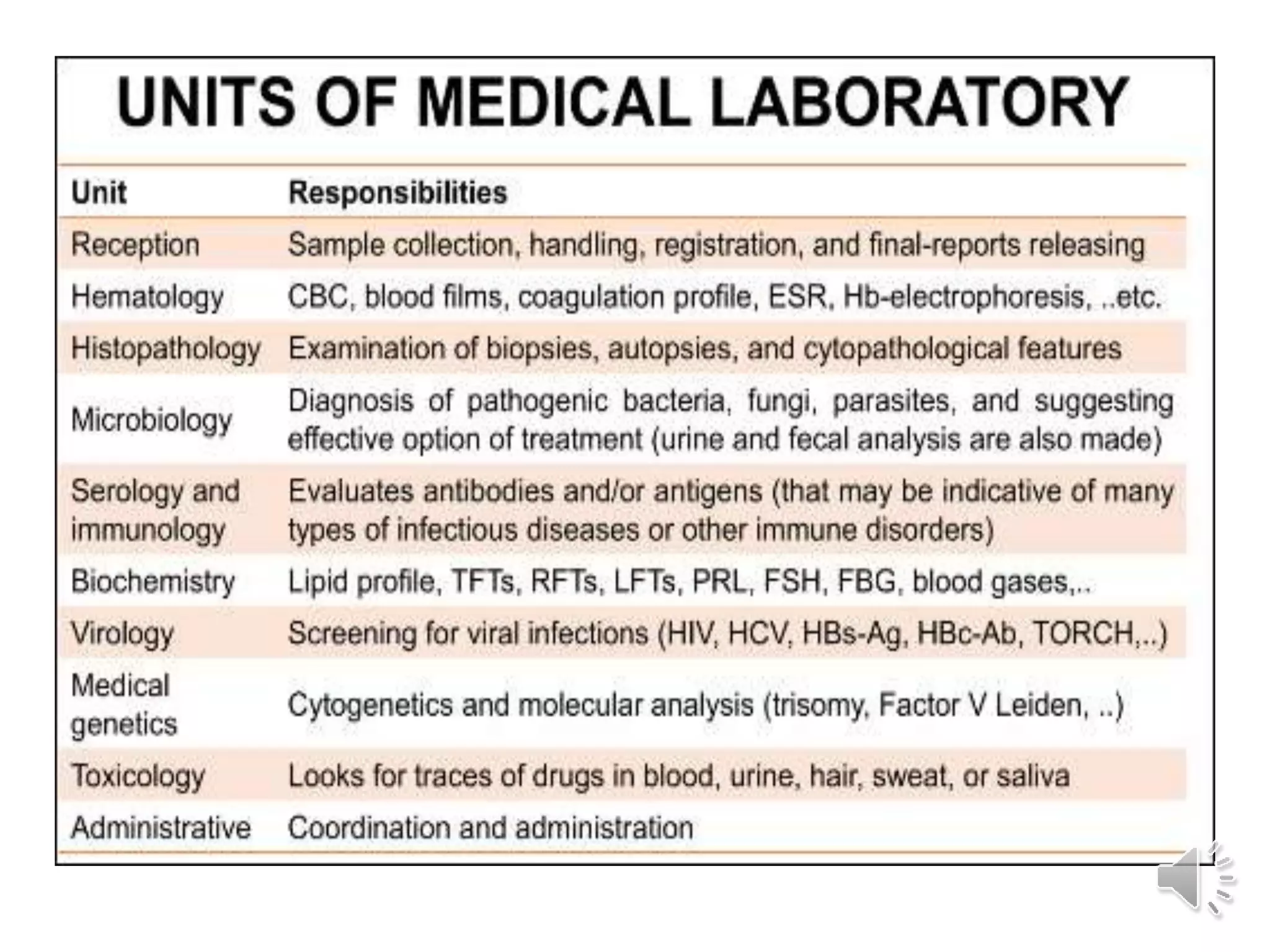
The medical laboratory workflow can be divided into three main phases: pre-analytical, analytical, and post-analytical. Samples first go through sample collection, transportation, and registration before being prepared for analysis. Quality controls are run regularly to ensure machine accuracy. Samples are then analyzed and results are validated before being reported to clinicians. Proper sample handling and a standardized workflow are important for reducing errors and turnaround time while maintaining quality.