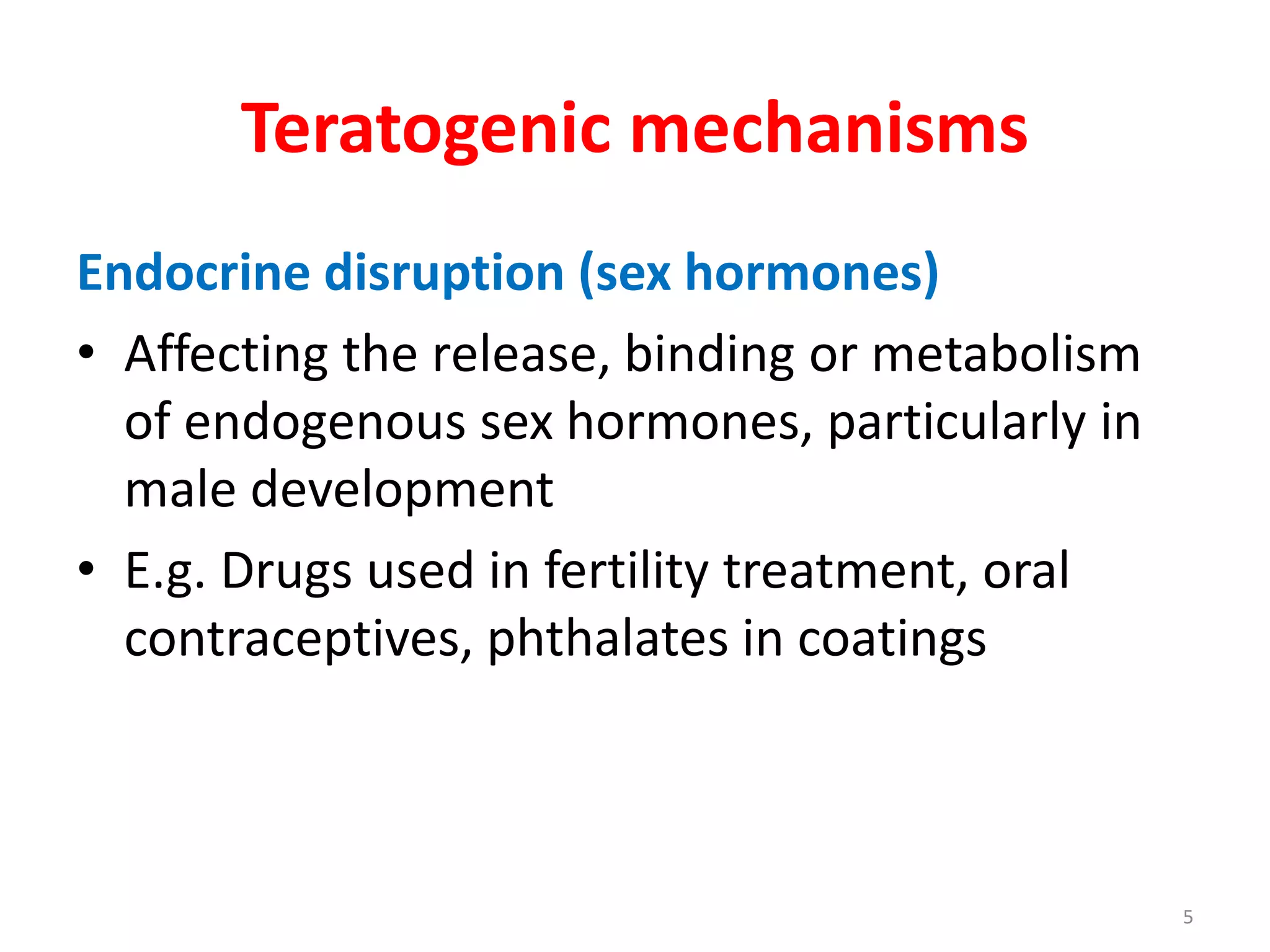
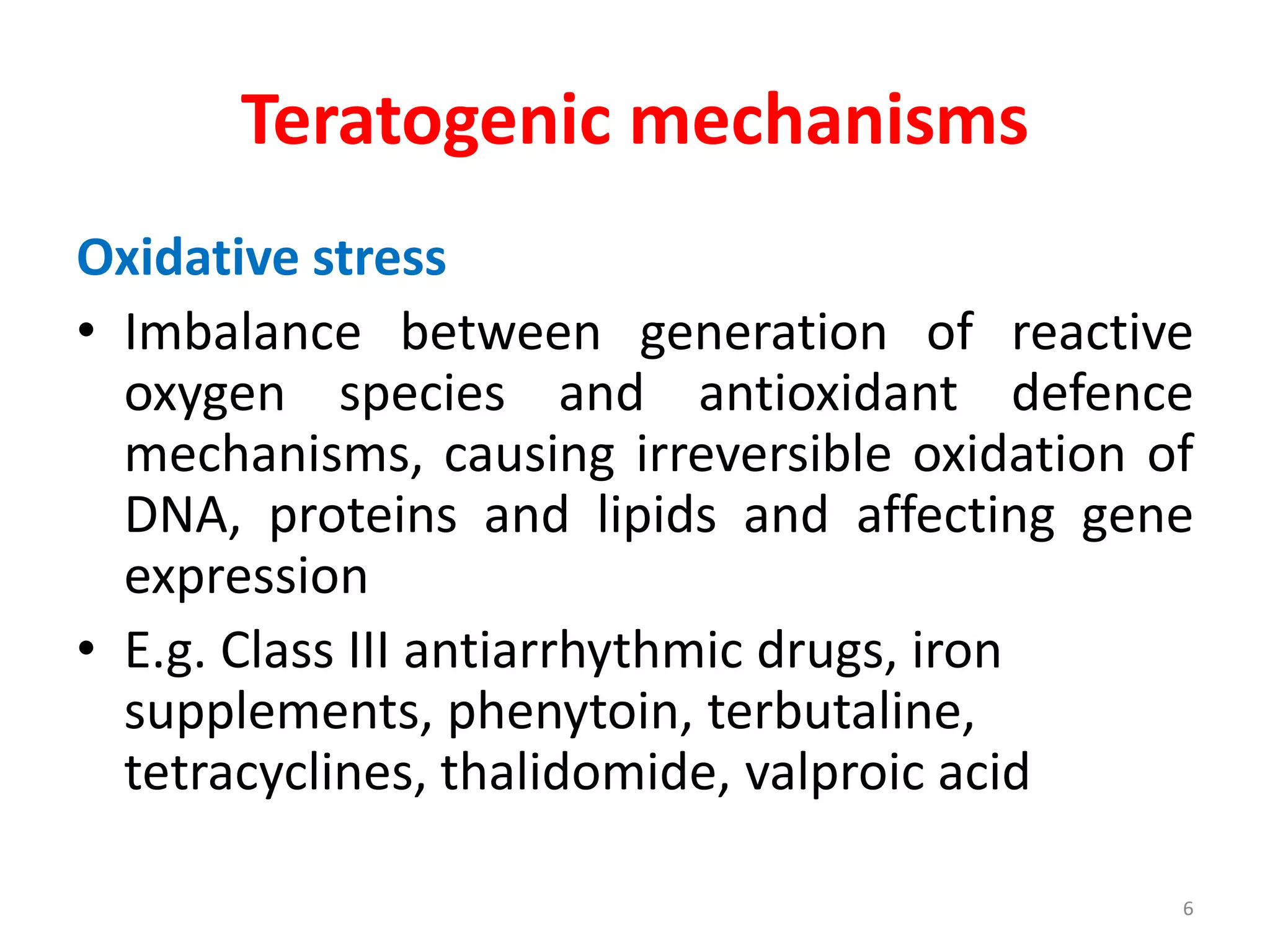
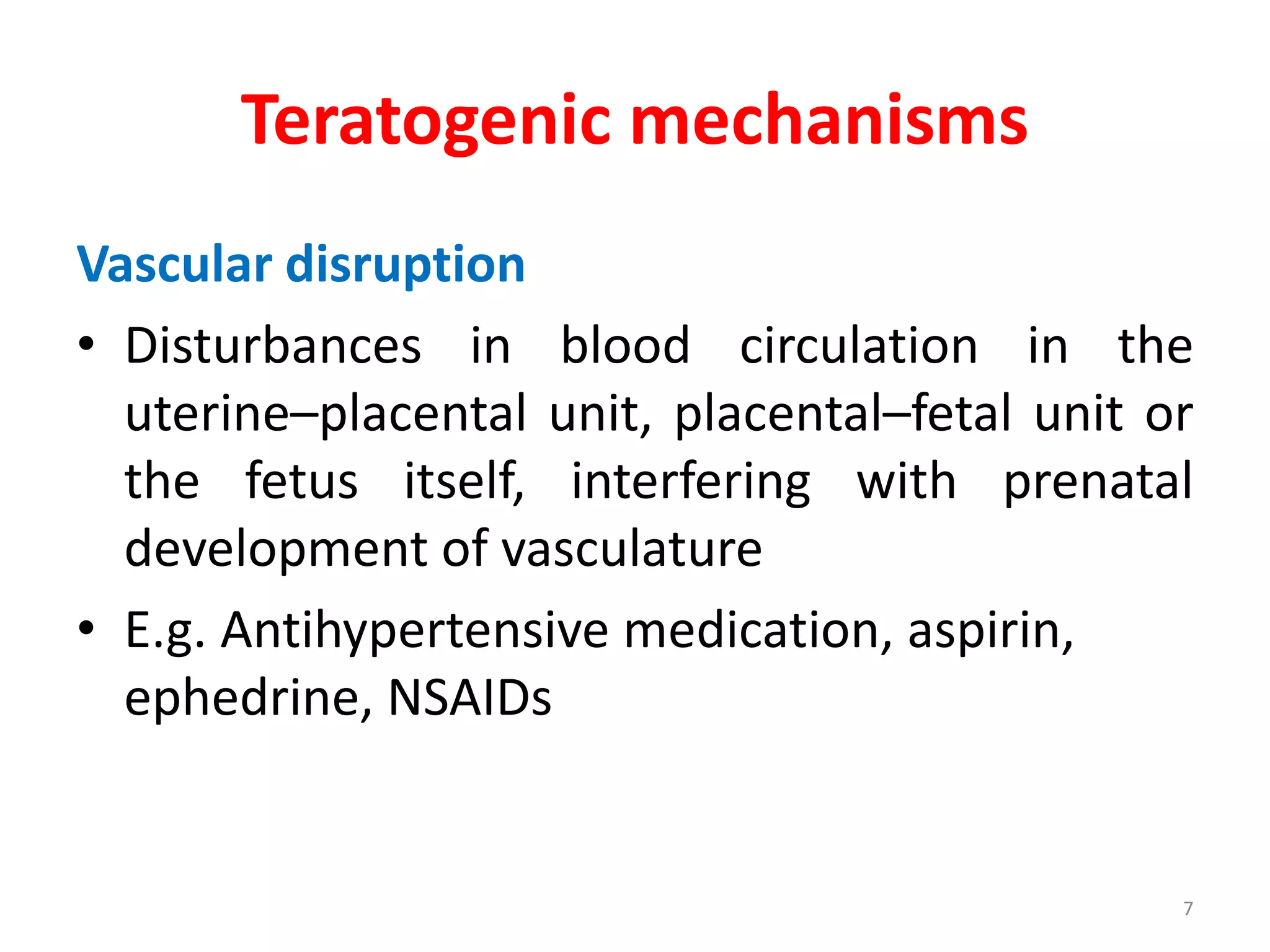
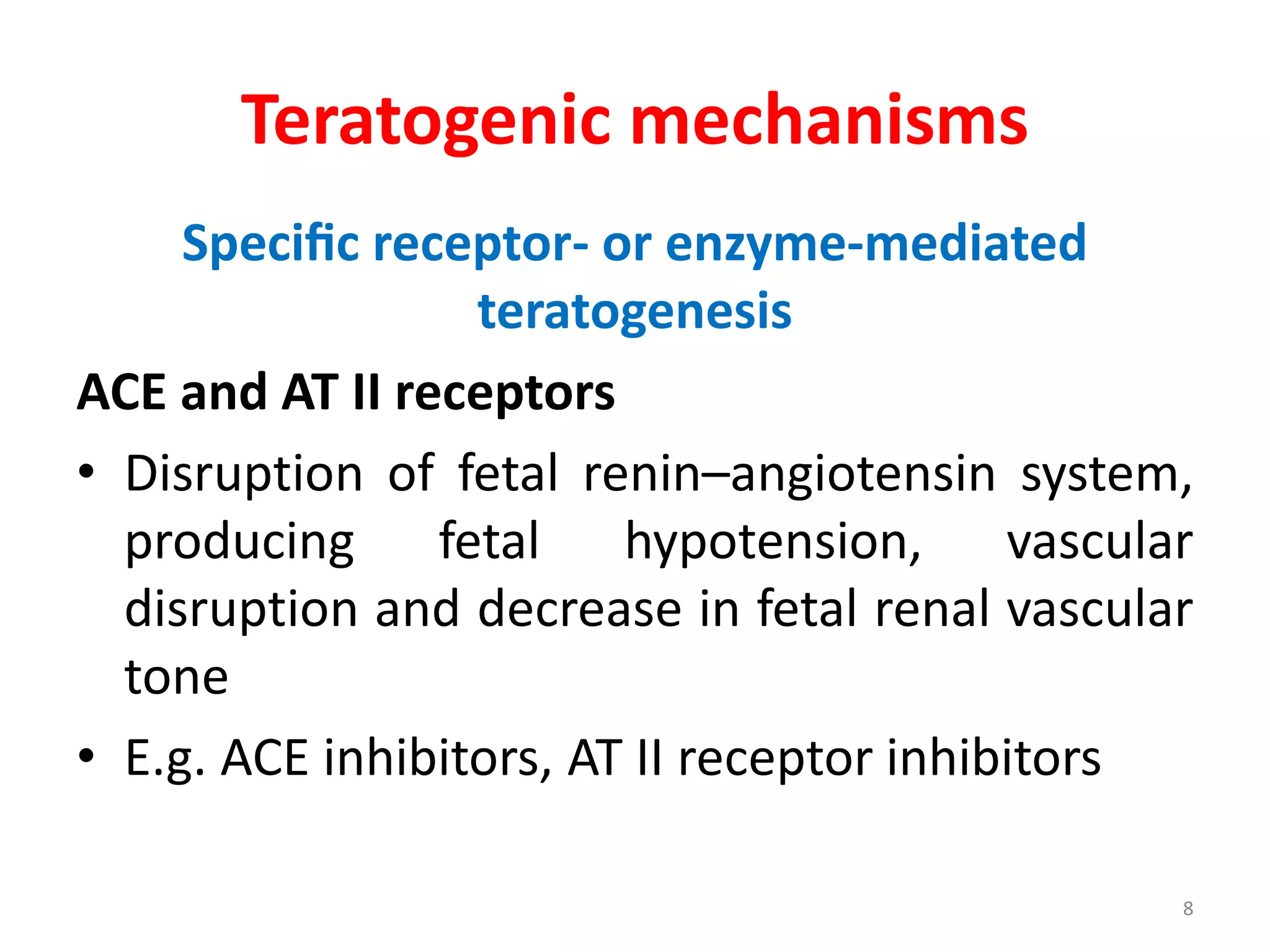
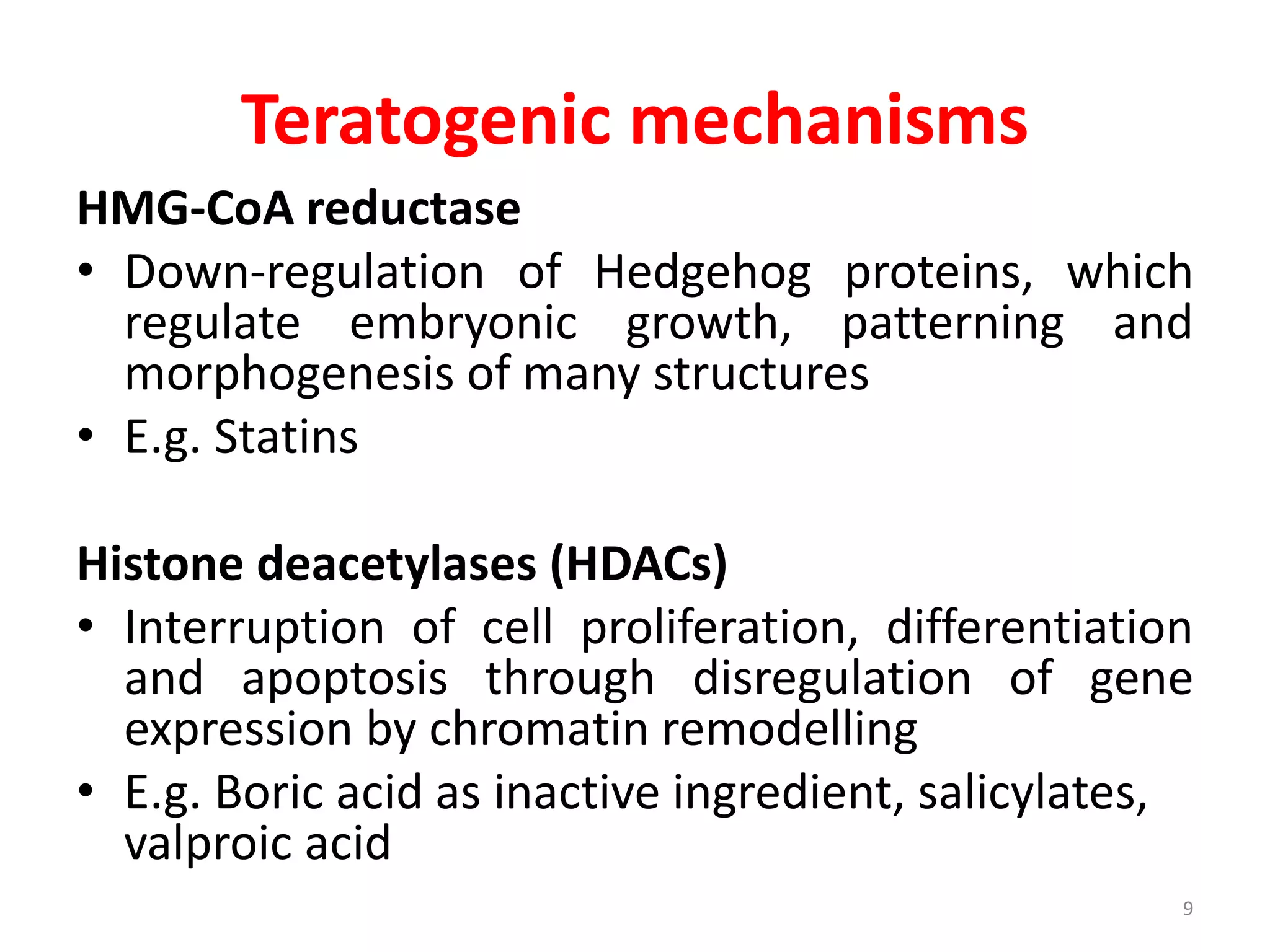
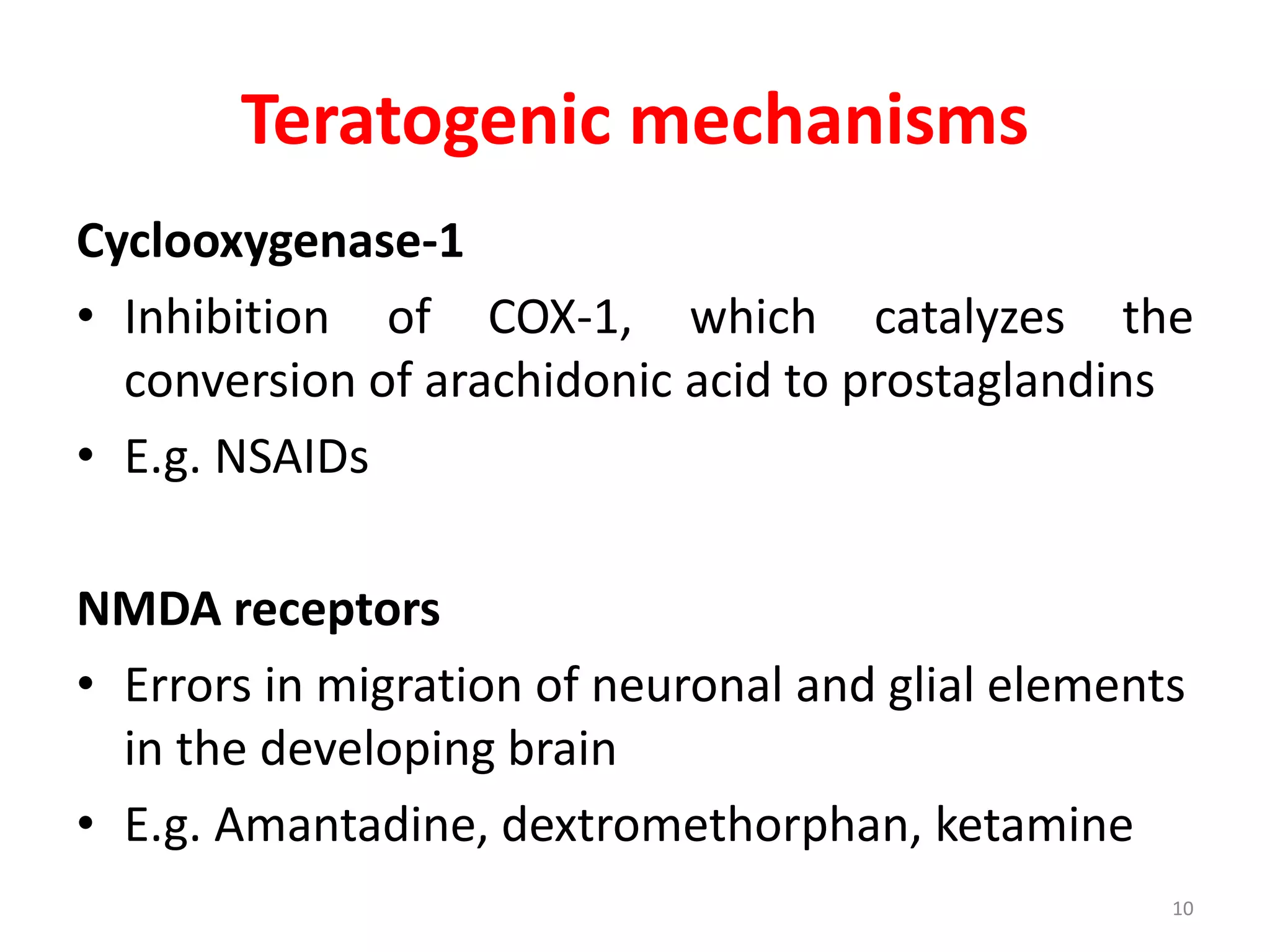
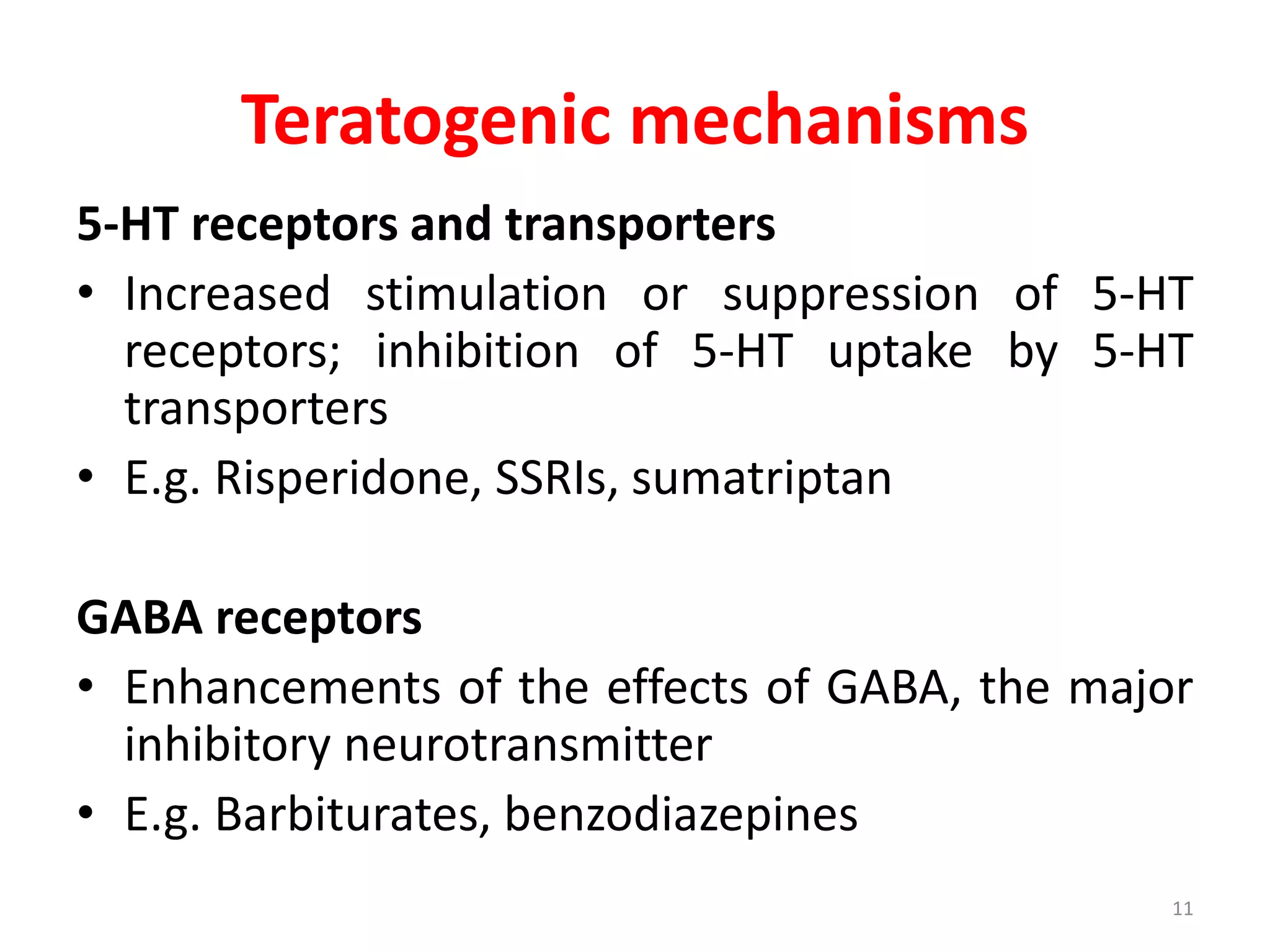
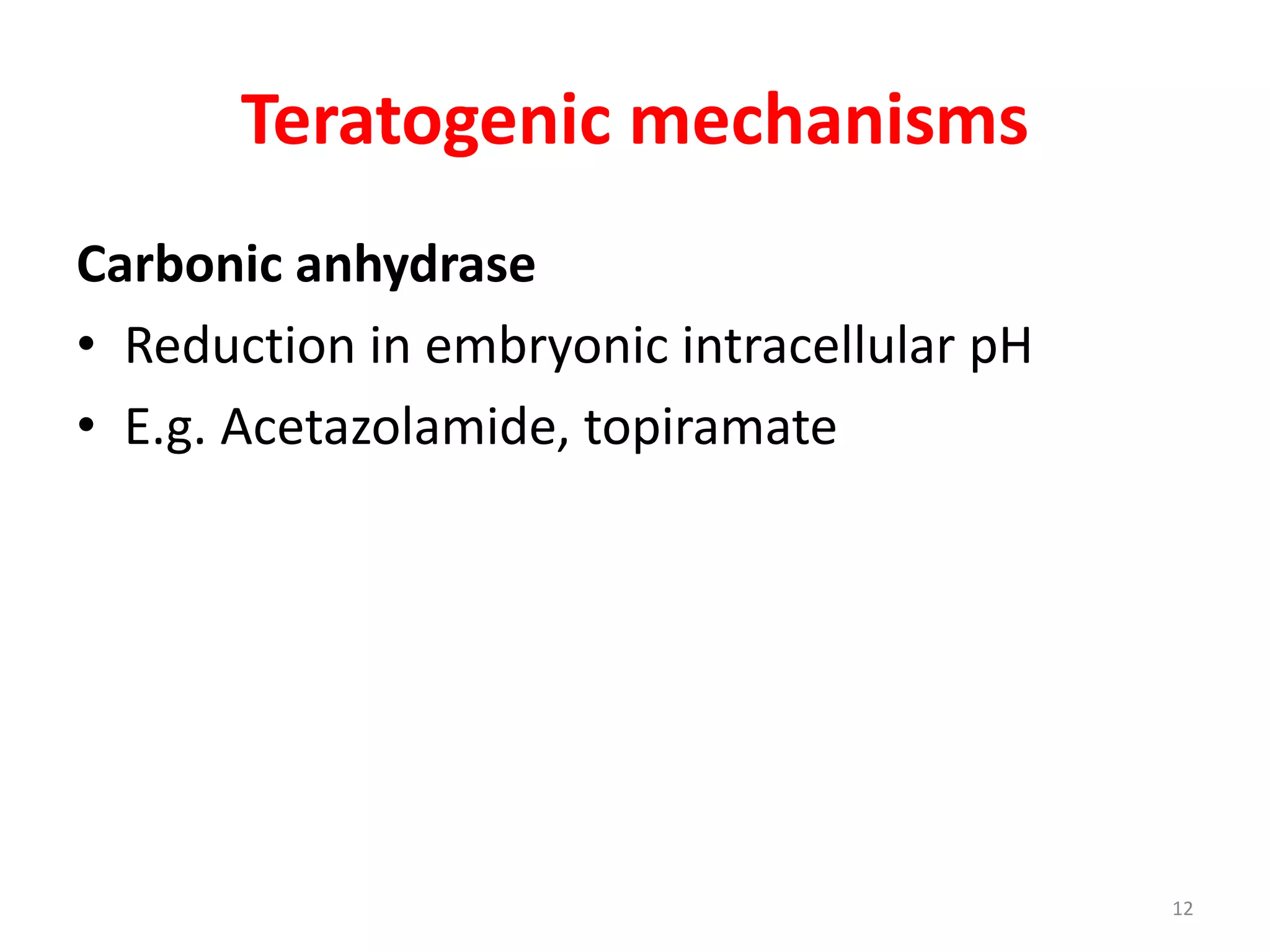
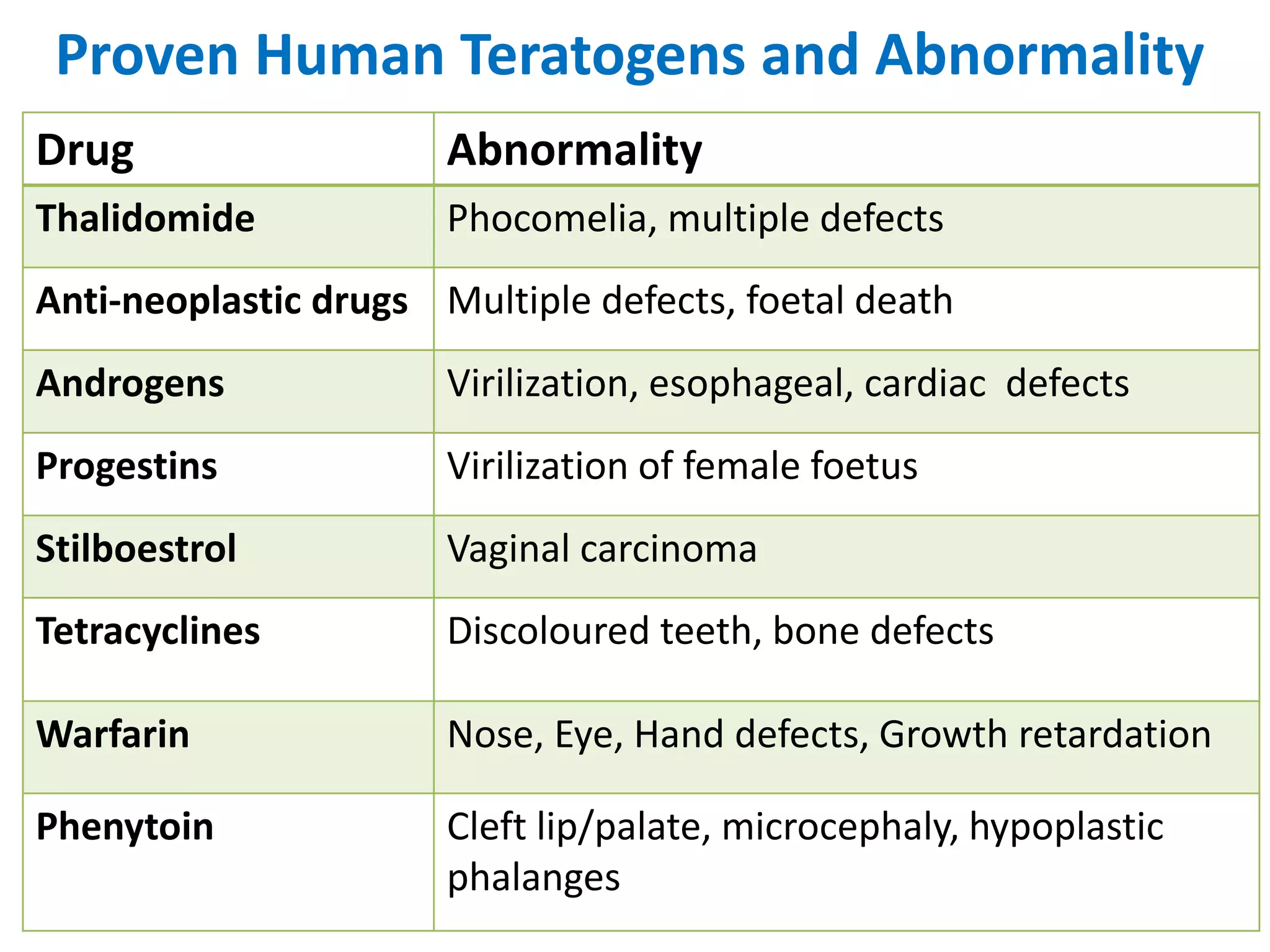
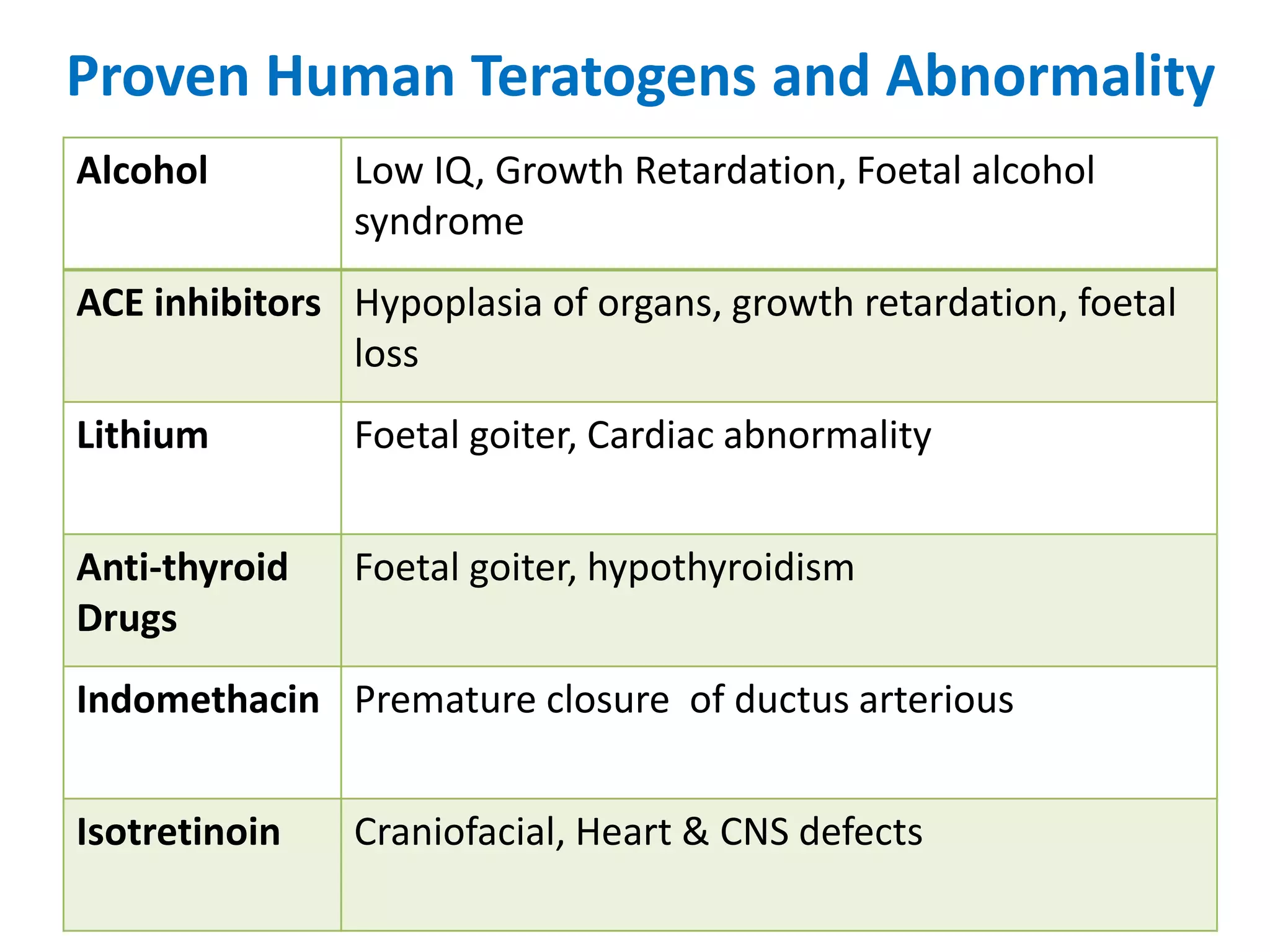
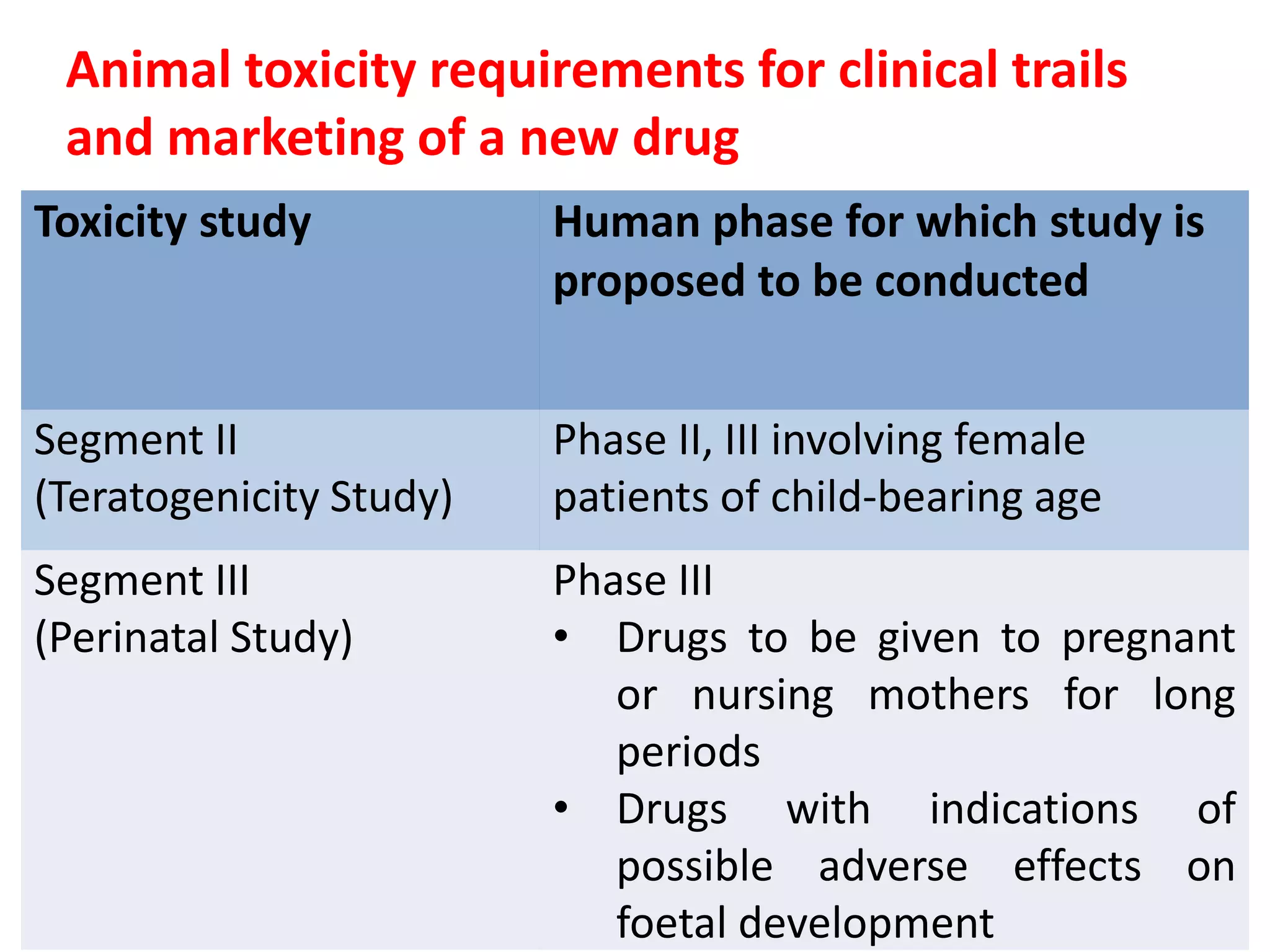
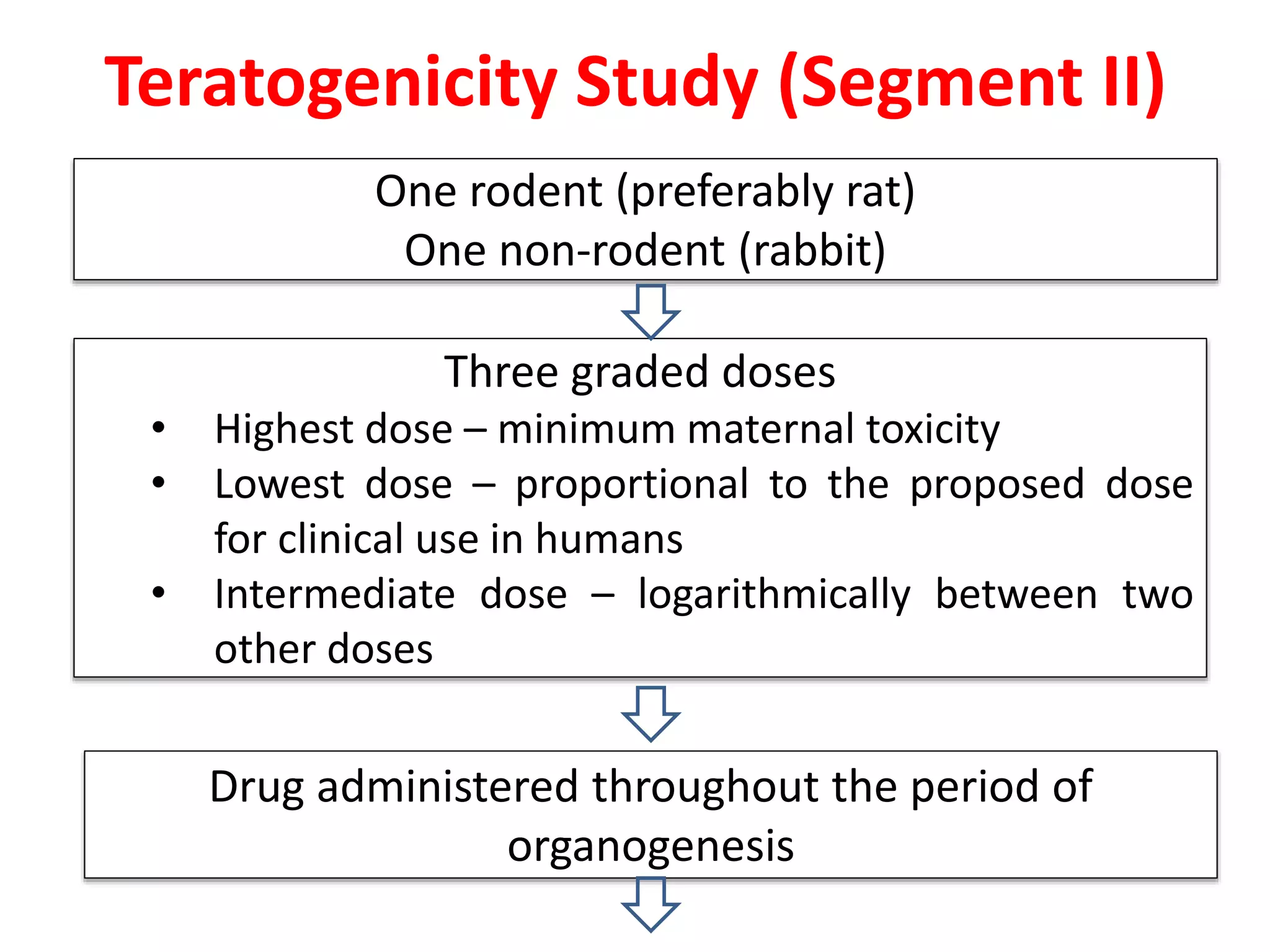
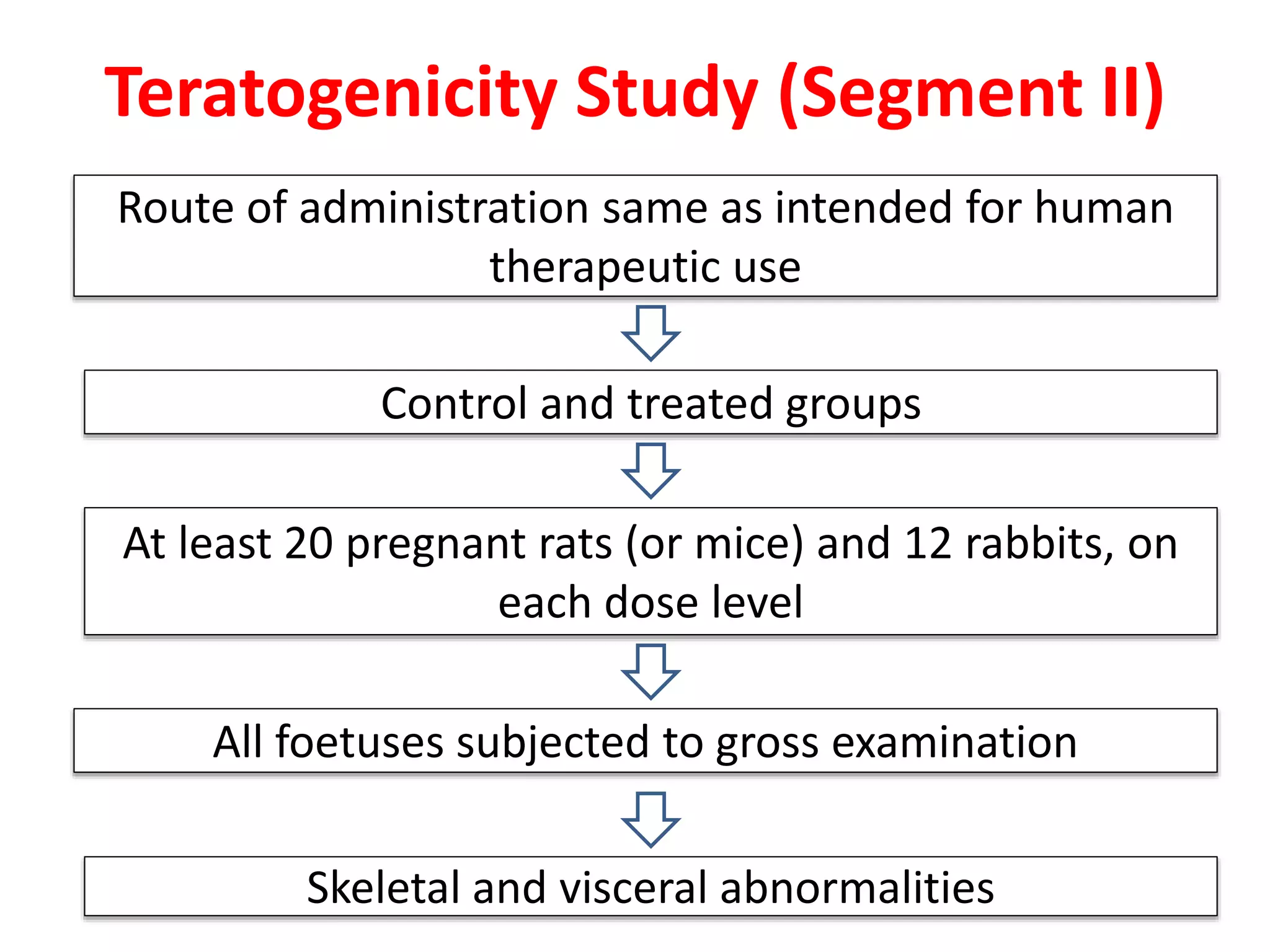
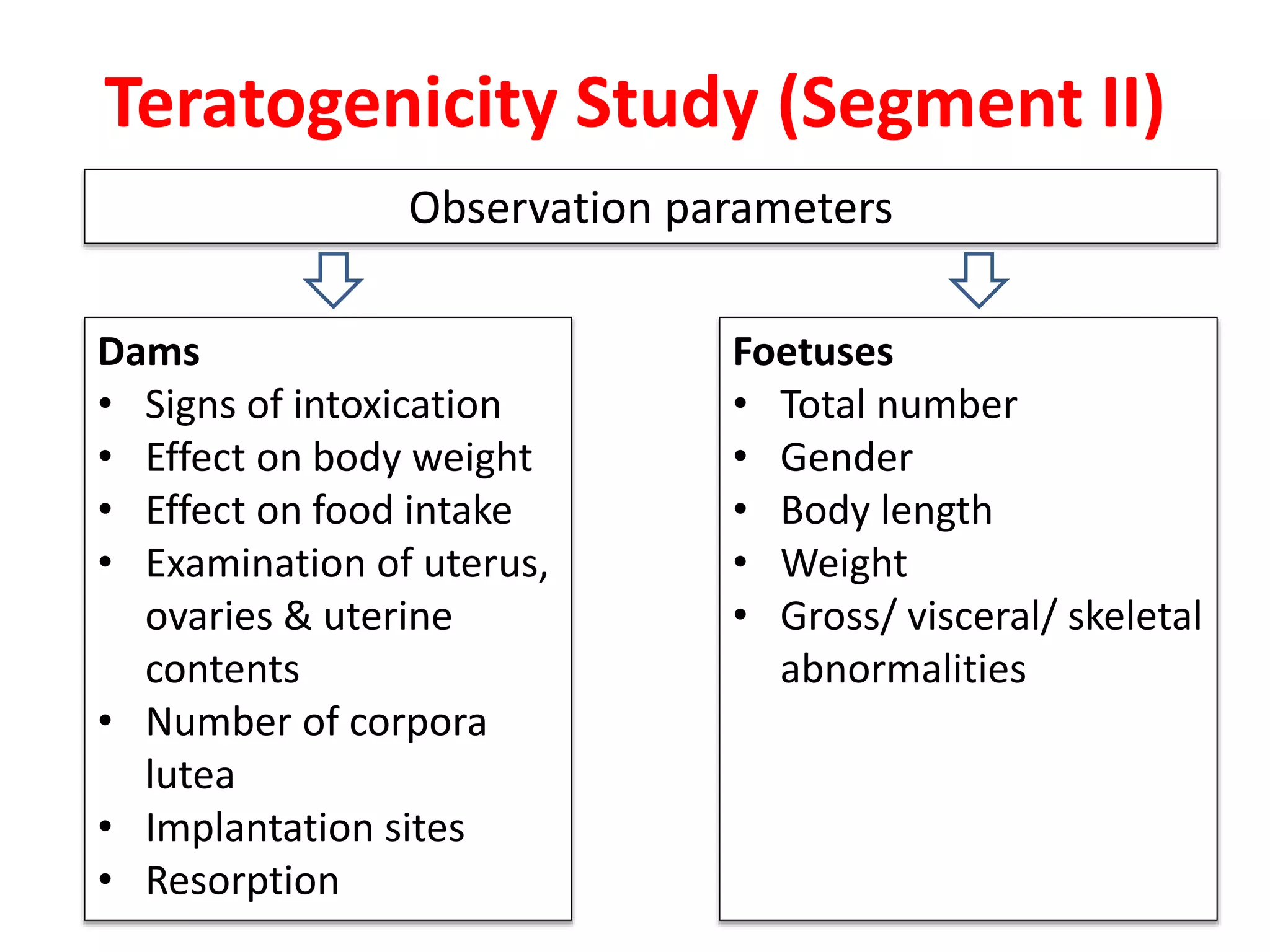
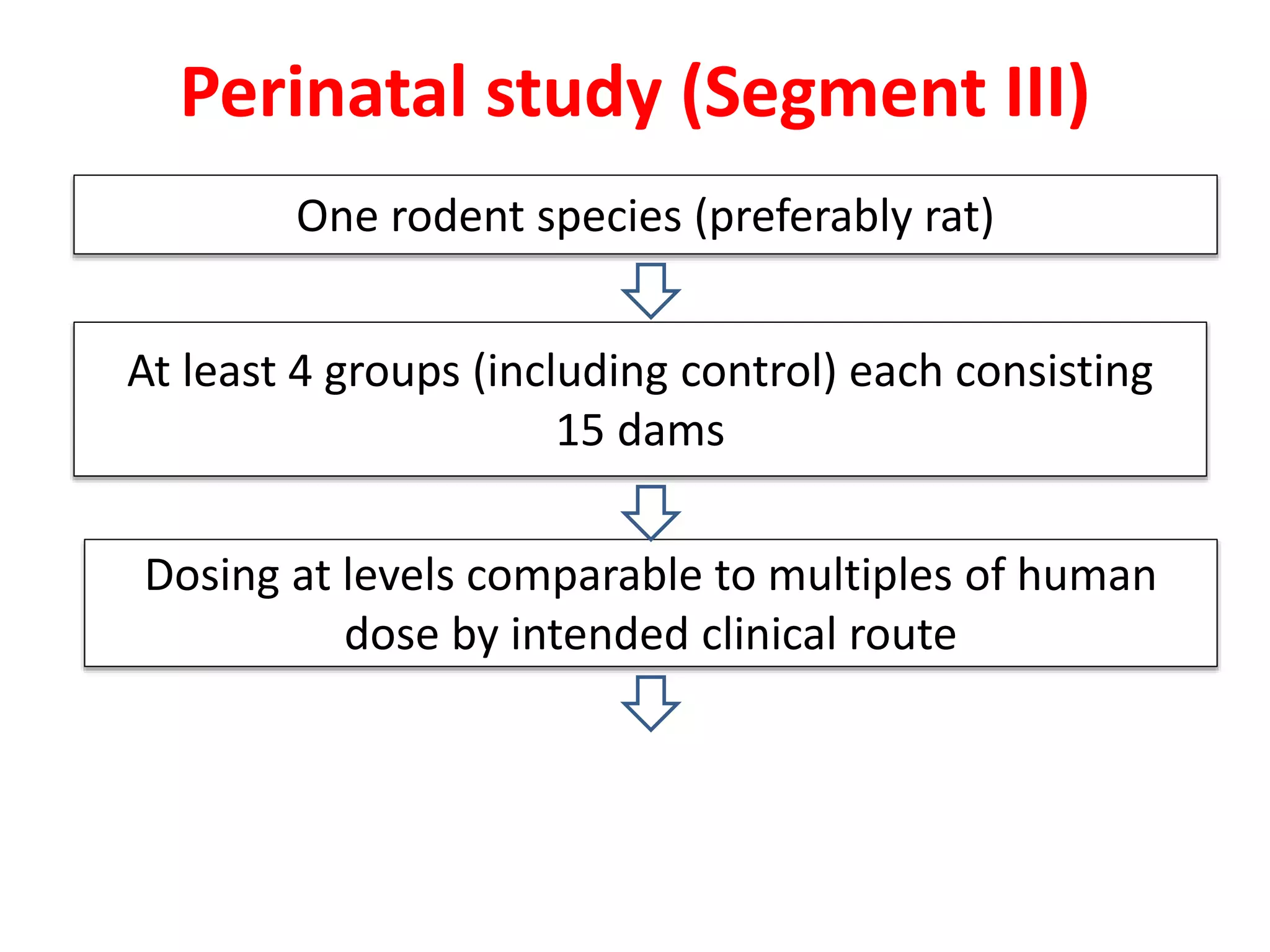
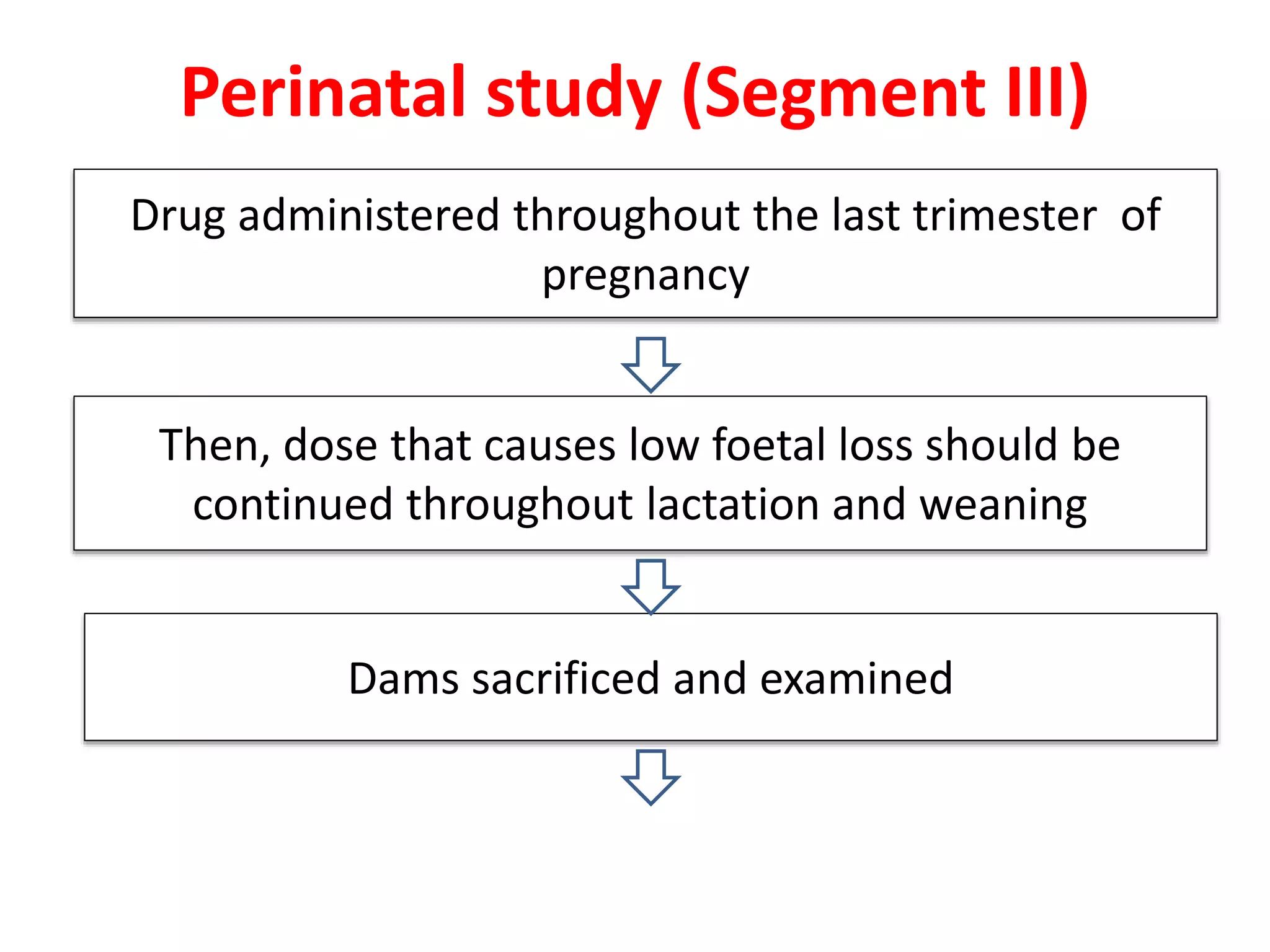
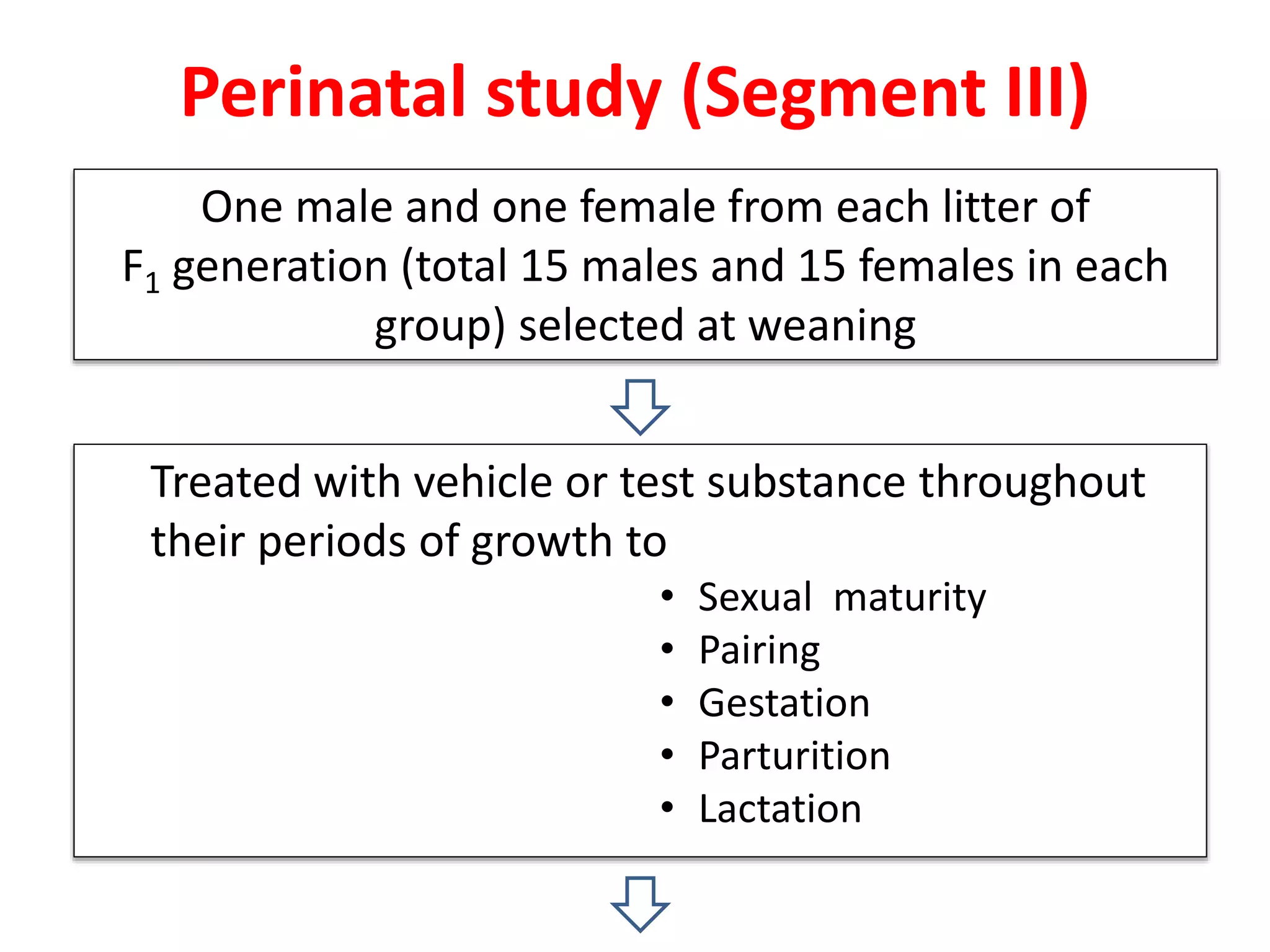
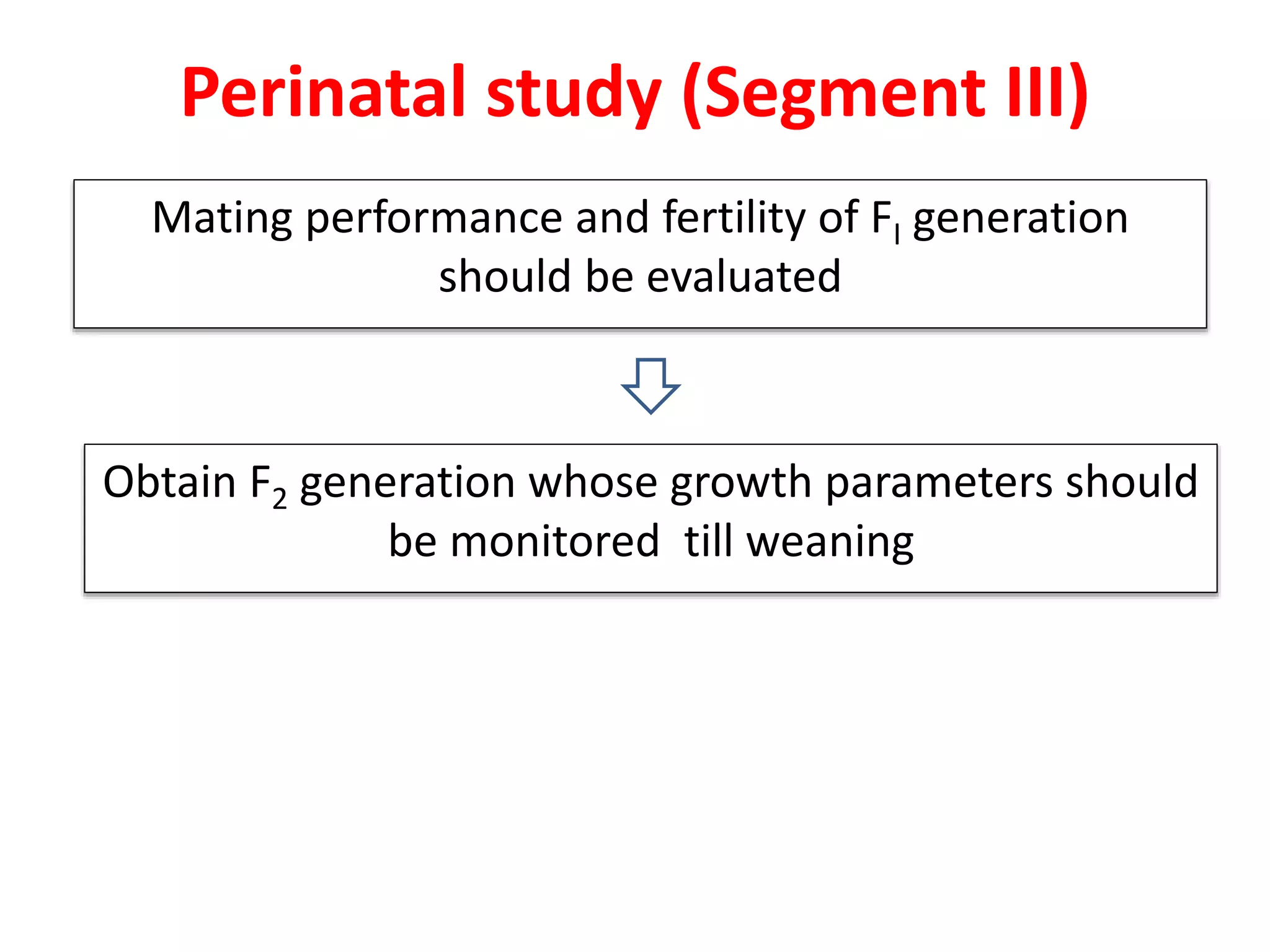
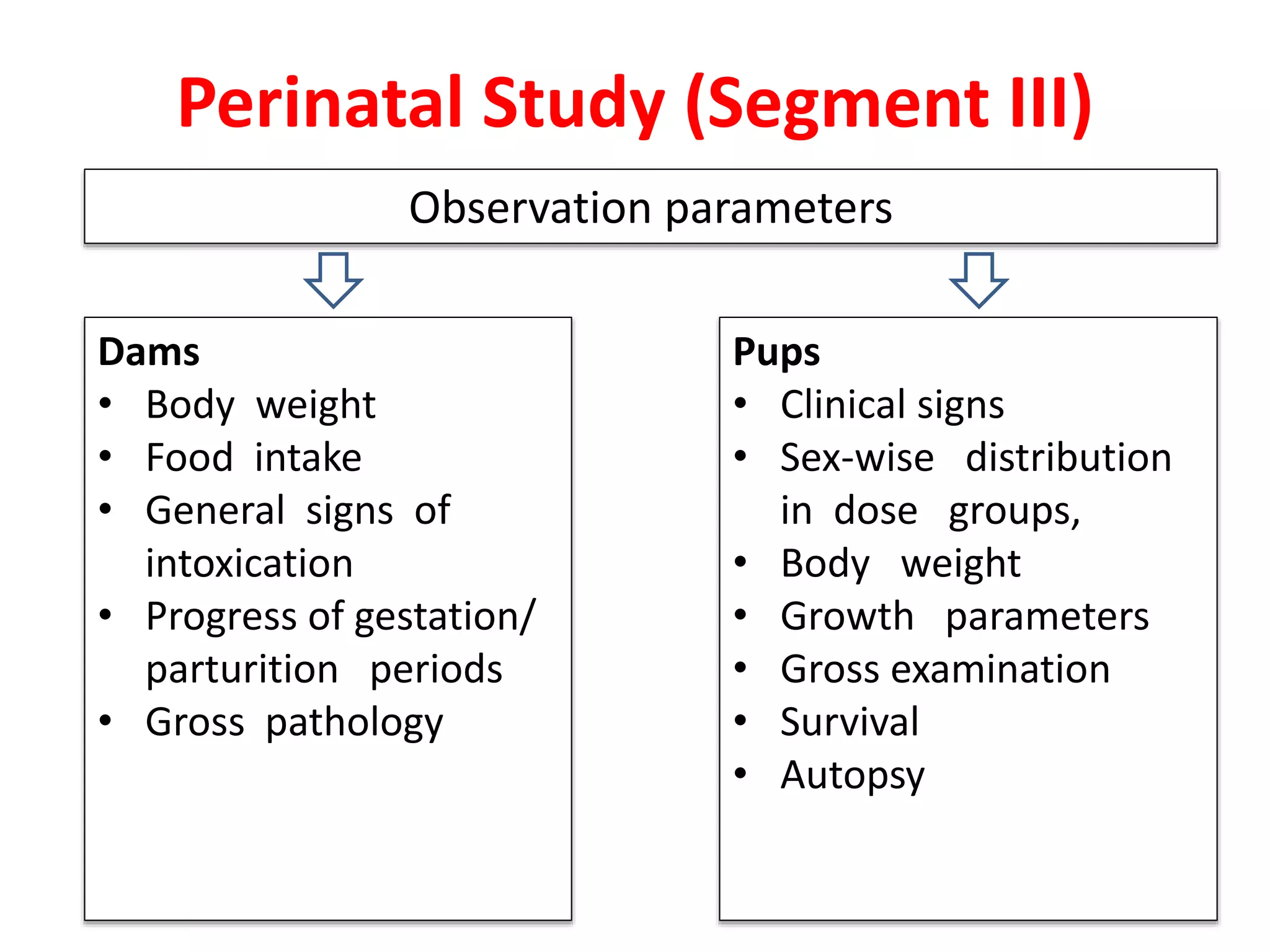
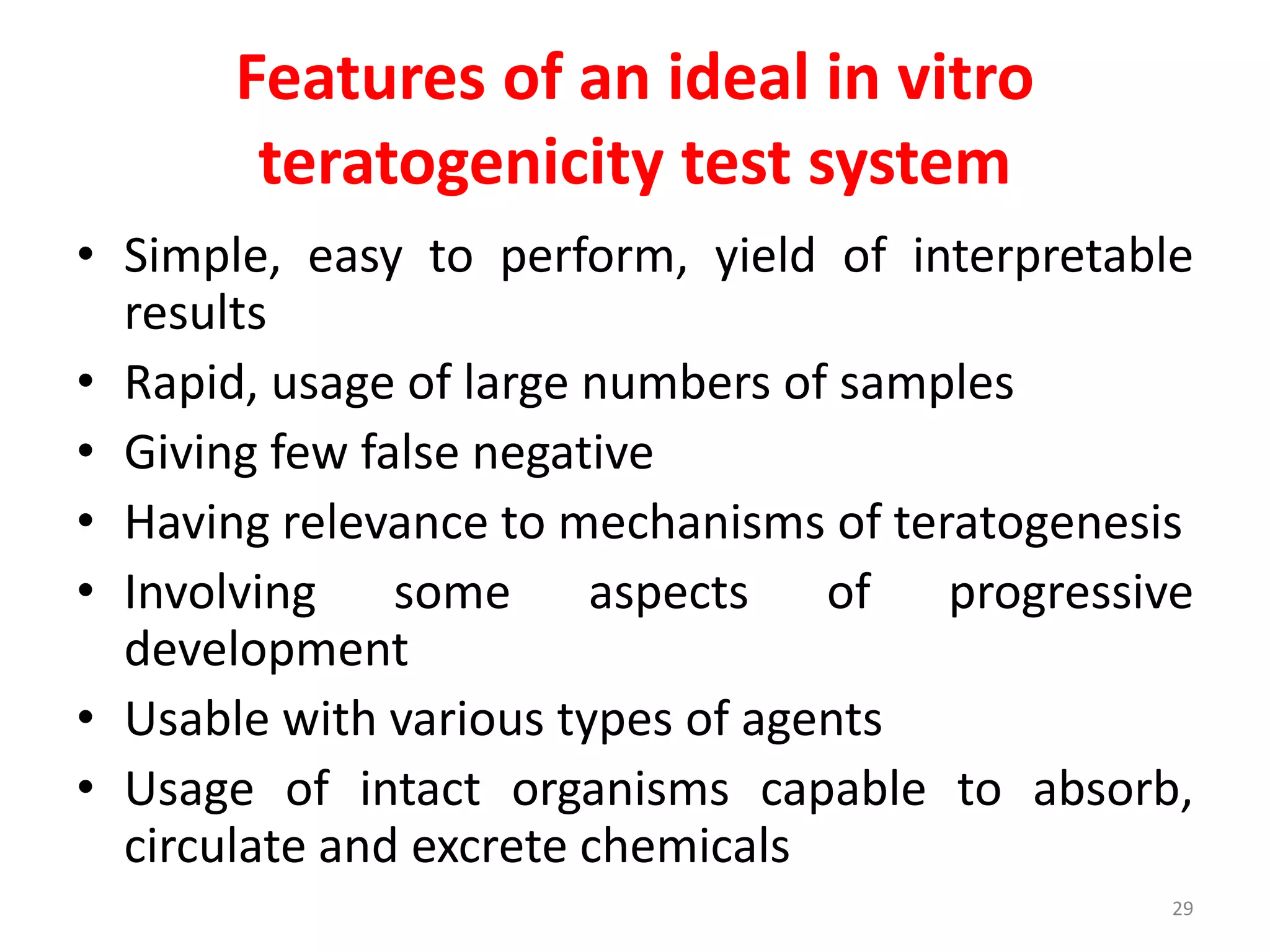
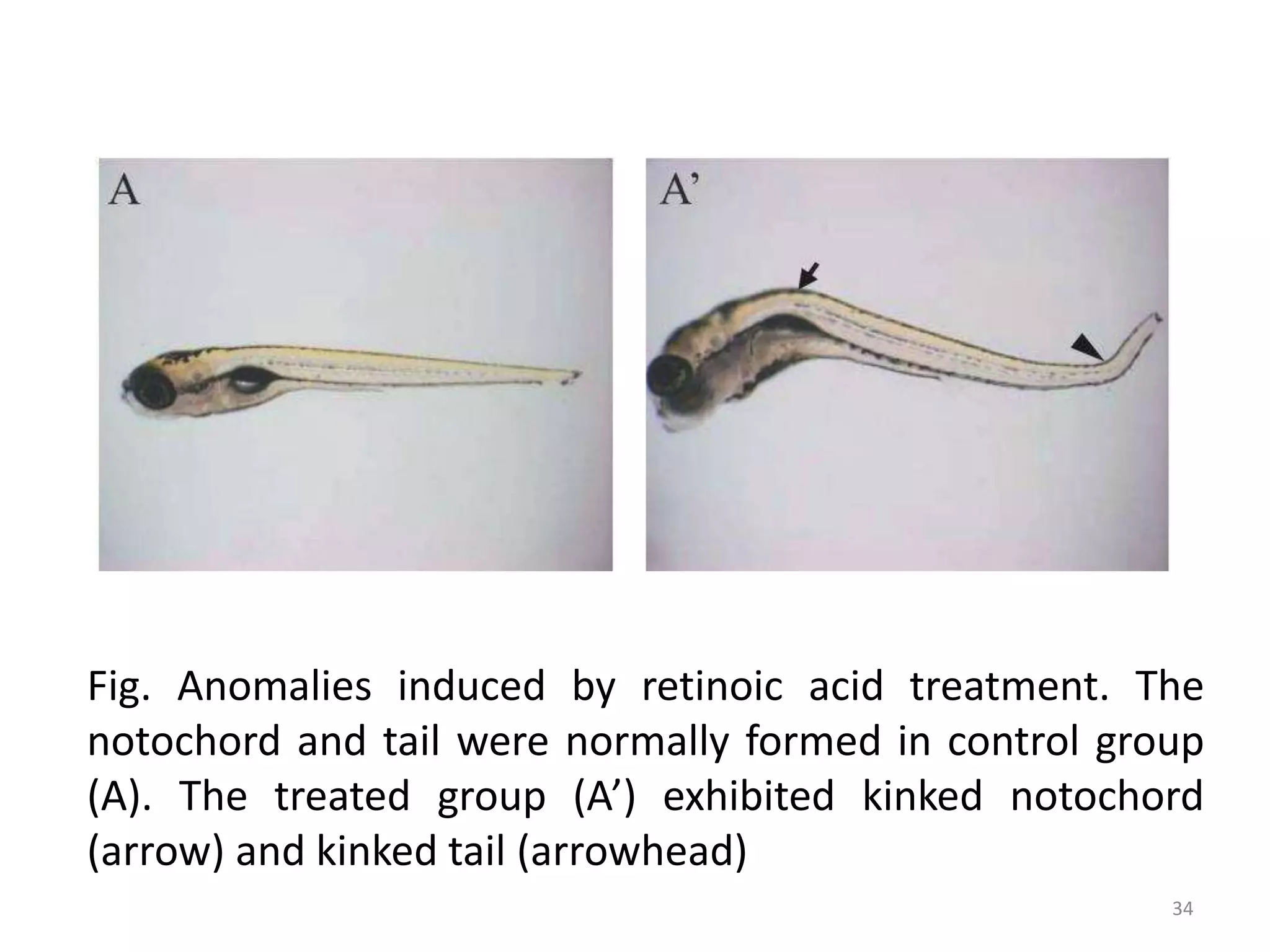
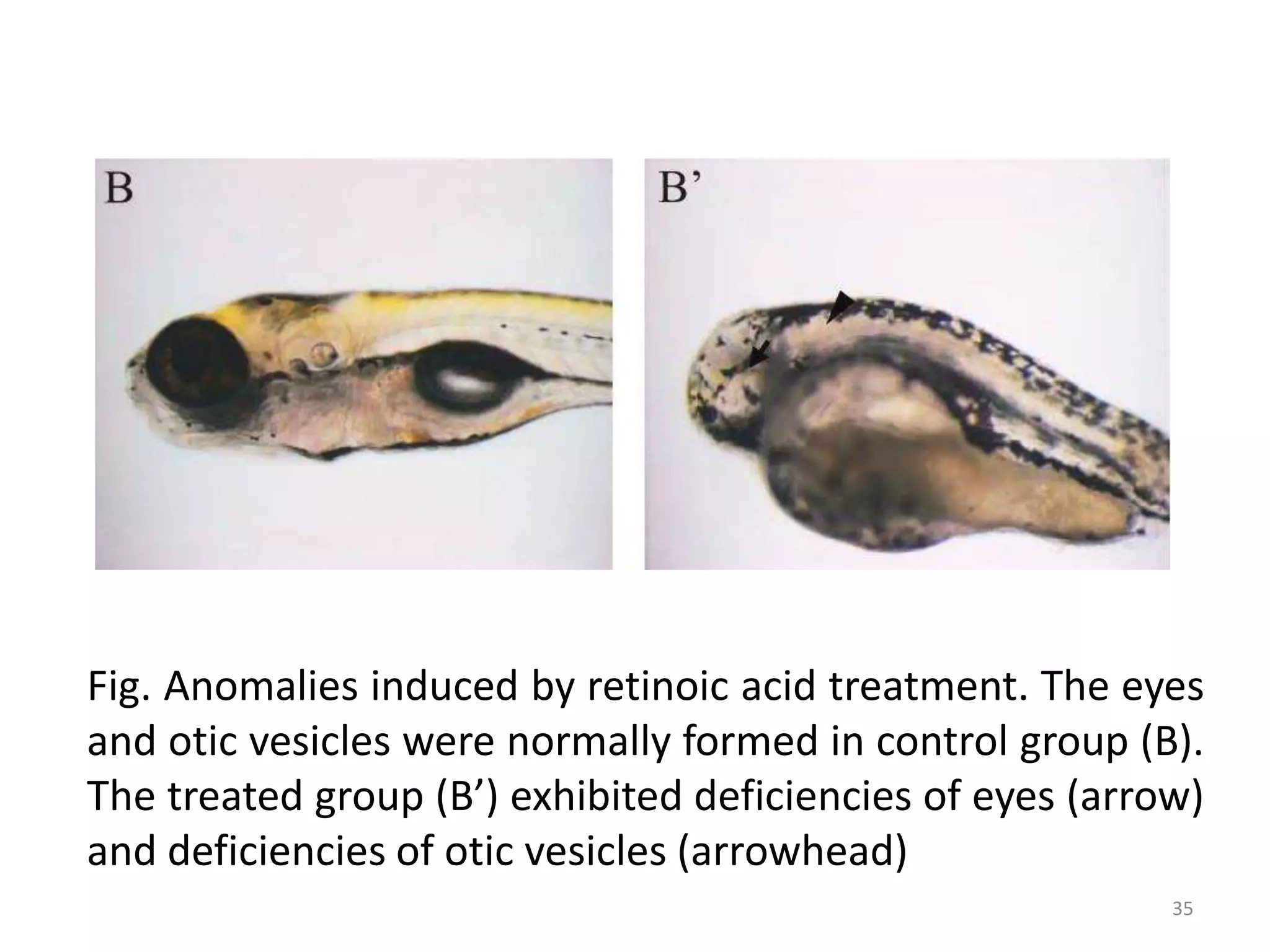
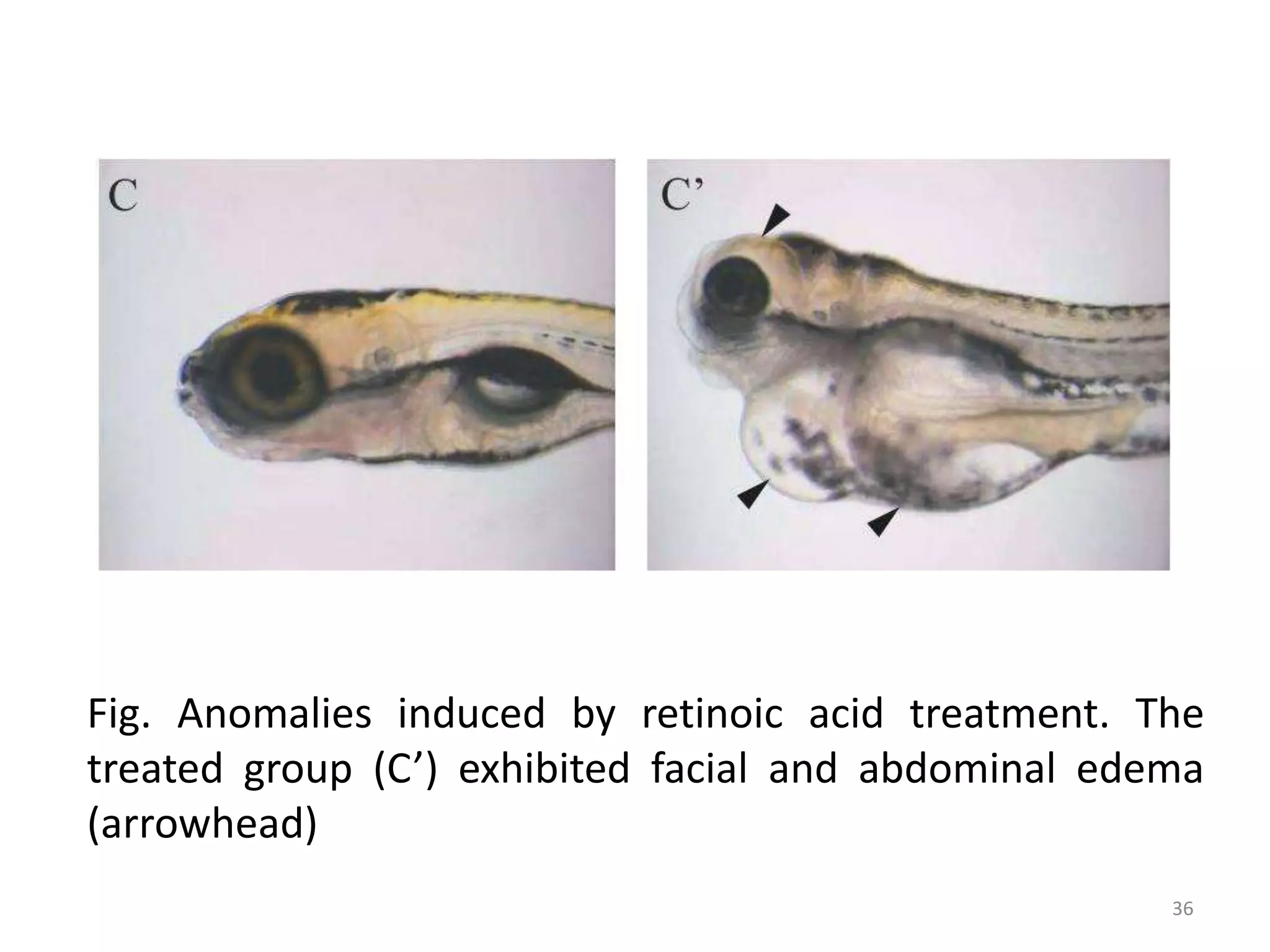
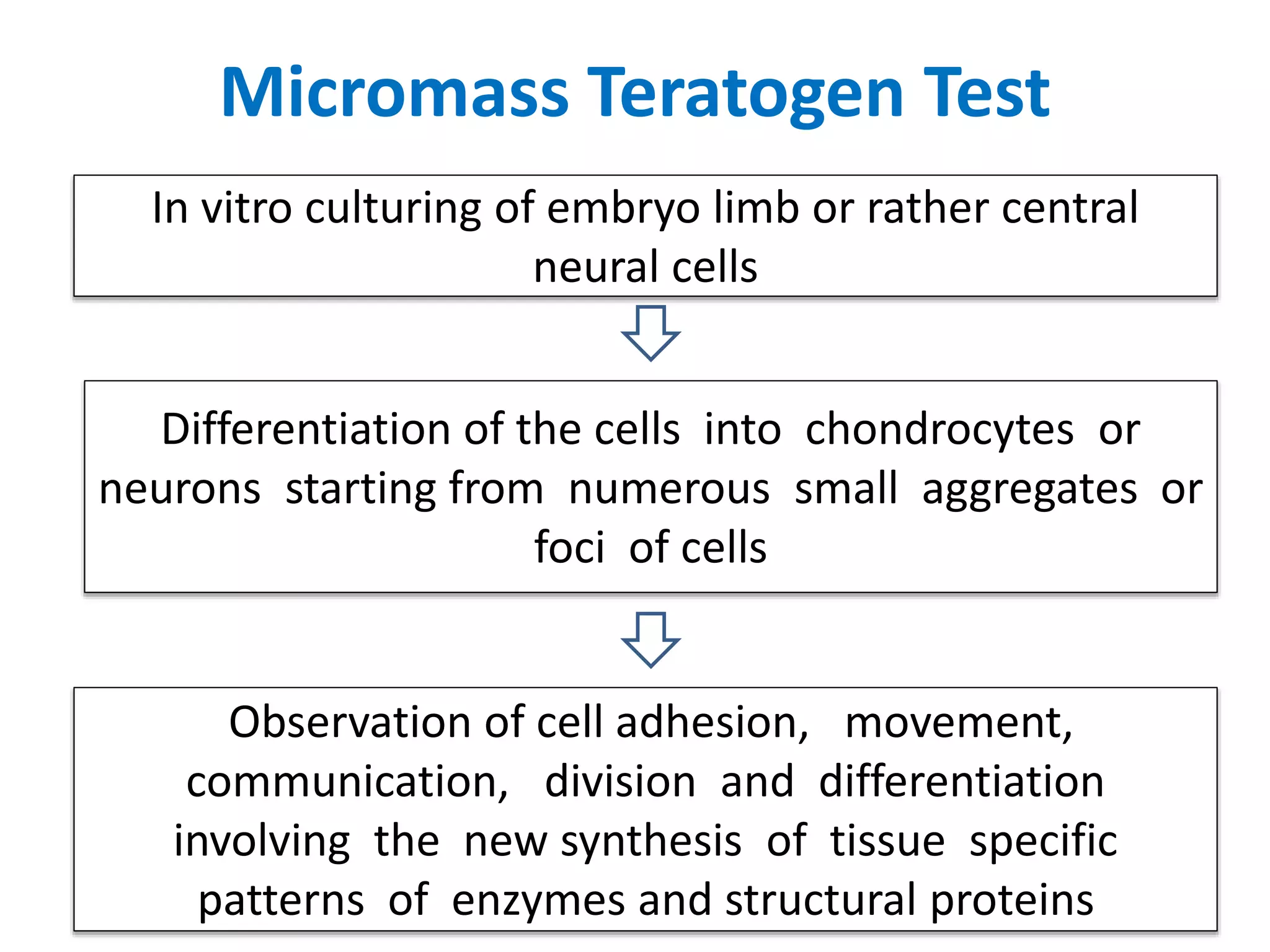
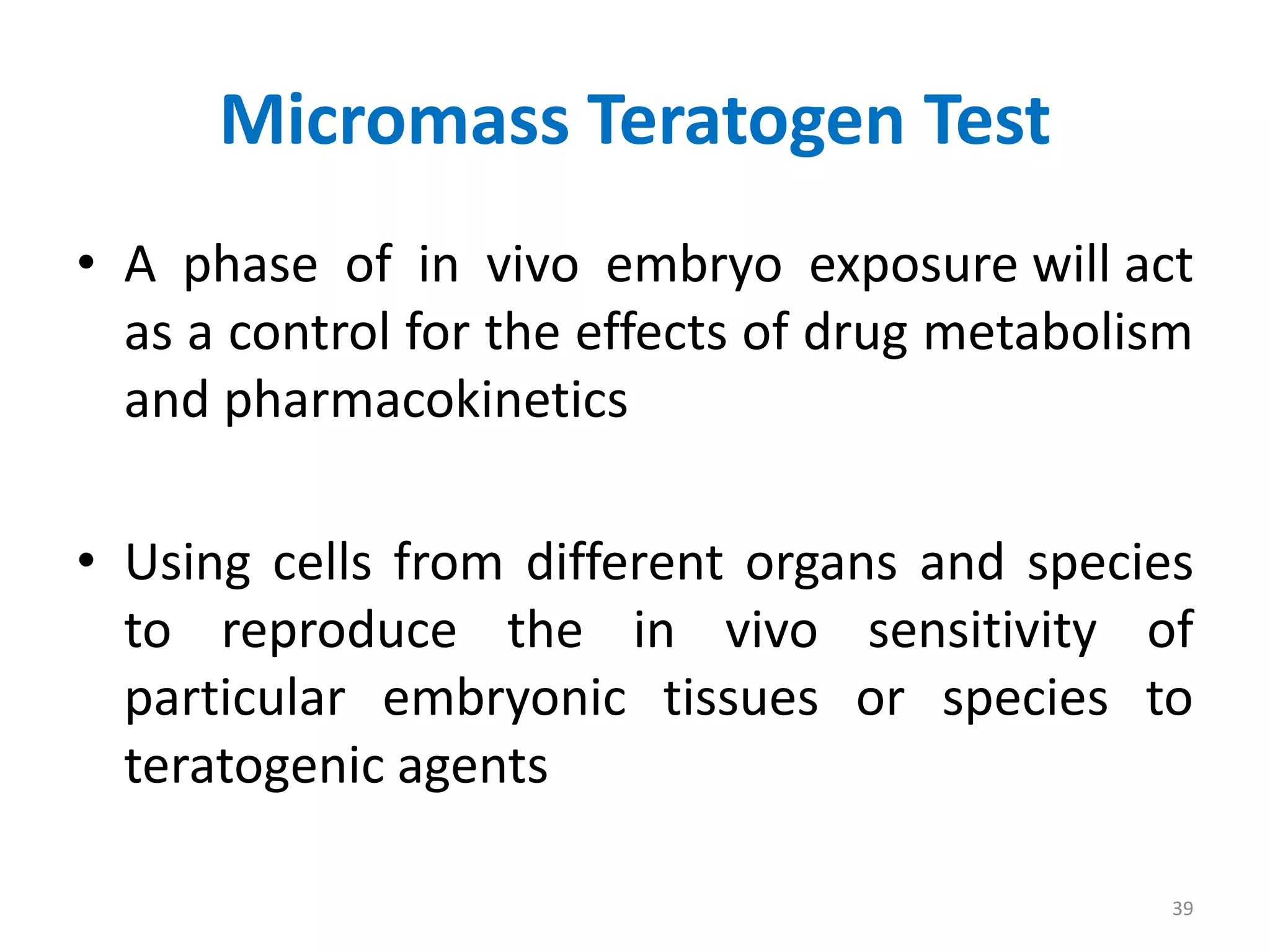
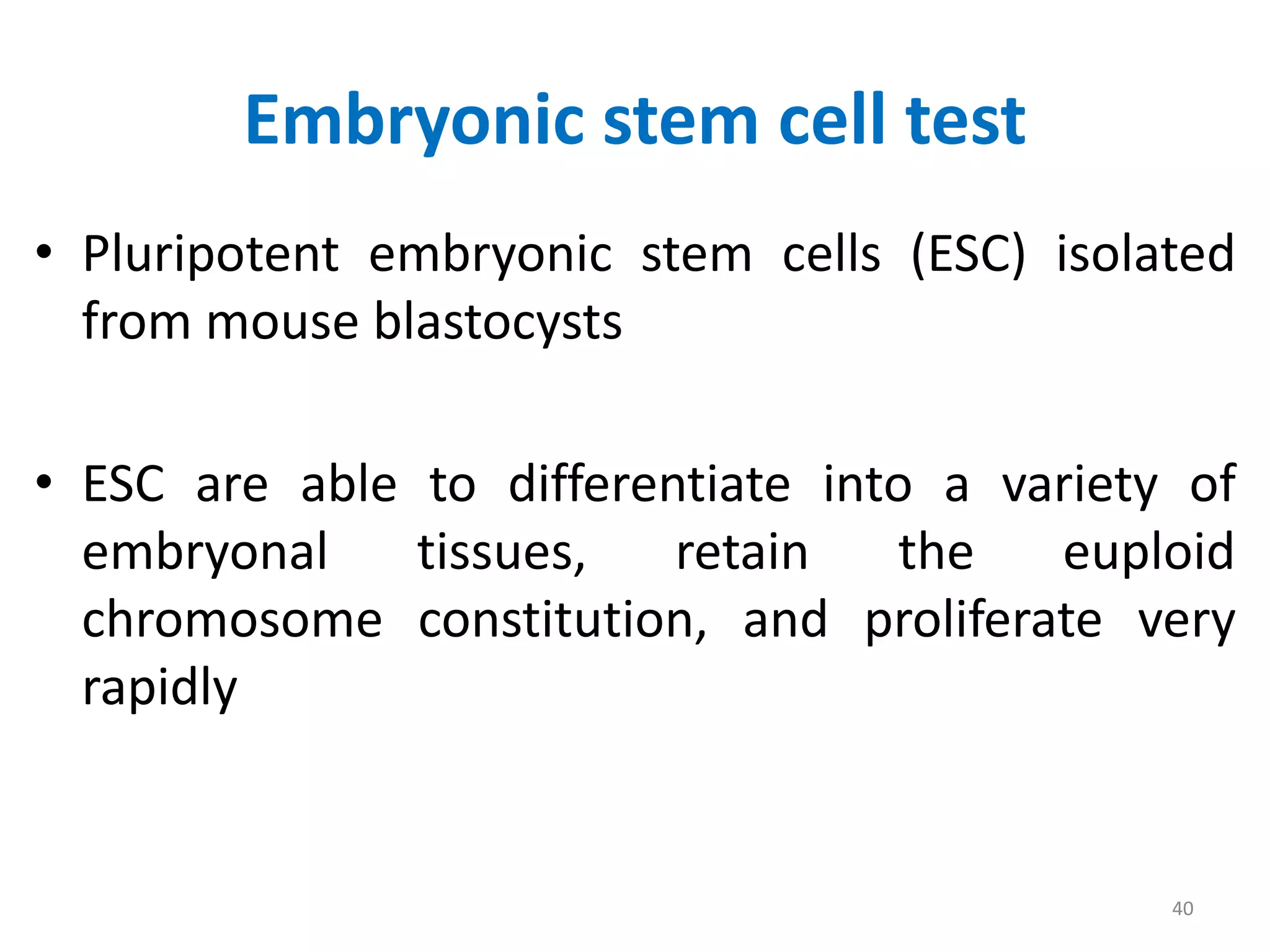
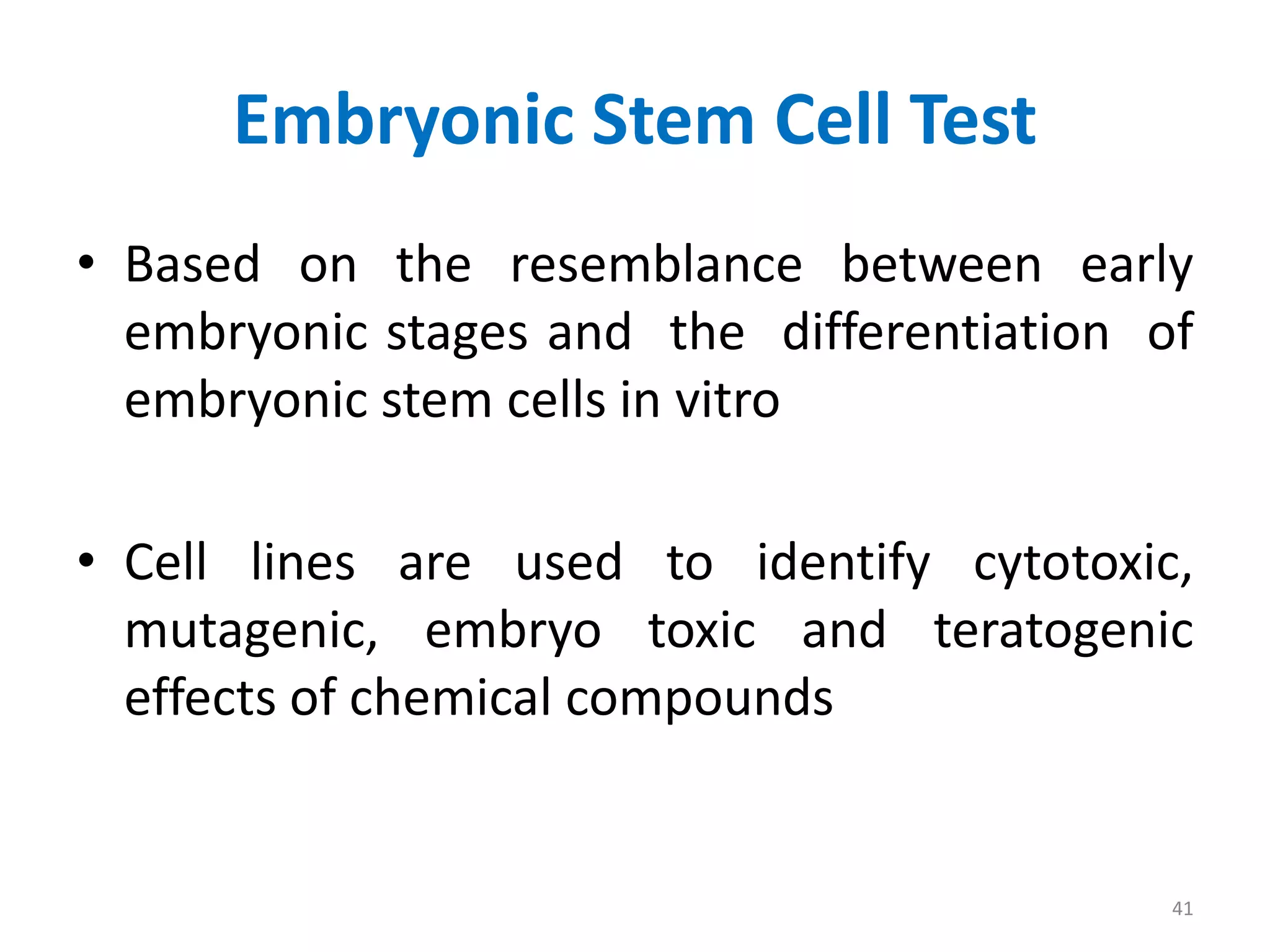
This document discusses various screening methods for detecting teratogenicity or the capacity of drugs to cause fetal abnormalities. It describes animal models like rat and rabbit studies that are used to test drugs during key stages of pregnancy. In vitro techniques like whole embryo culture, micromass assays, and stem cell tests are summarized as alternatives that can reduce animal use. The mechanisms by which certain proven human teratogens cause defects are outlined. Regulatory guidelines for preclinical teratogenicity studies and testing categories for drug use in pregnancy are also summarized.