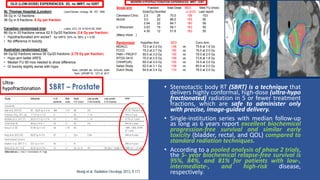
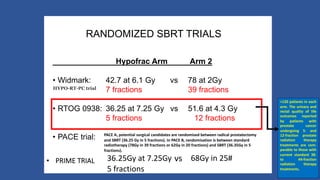
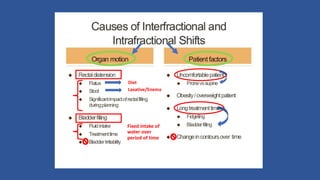
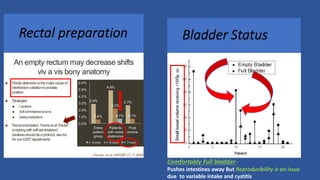
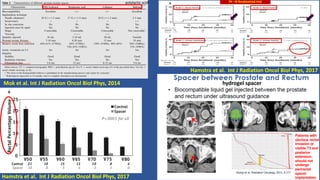
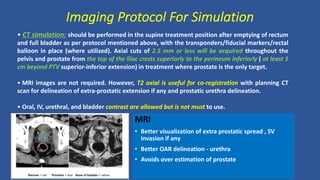
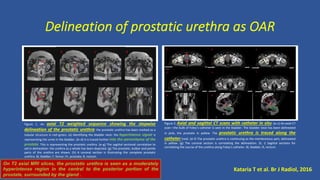
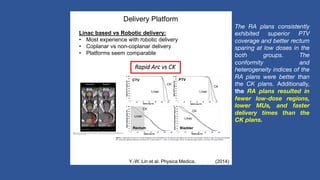
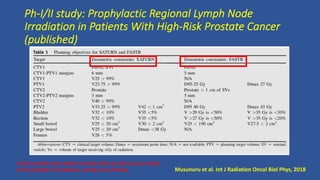
This document discusses treatment options for localized prostate cancer, highlighting the effectiveness and cost-efficiency of Stereotactic Body Radiation Therapy (SBRT) compared to traditional methods like surgery and standard radiotherapy. It emphasizes the benefits of hypofractionation due to prostate cancer's low alpha/beta ratio, enabling higher doses per fraction while maintaining toxicity levels. The document also outlines patient selection criteria for SBRT, emphasizing careful preparation and precise imaging for optimal treatment outcomes.